To describe clinical manifestations, antecedents, comorbidities and associated treatments, imaging findings, and follow-up in patients with posterior reversible encephalopathy syndrome.
MethodsA retrospective, descriptive analysis of admitted patients was performed between June 2009 and May 2014 in a third-level care hospital. We evaluated age, sex, comorbidities, symptoms, values of blood pressure at admission, renal function, medication and time elapsed until the disappearance of symptoms.
ResultsThirteen patients were included. In all, 77% of them had a history of hypertension at baseline and 85% had impaired renal function. The most prevalent comorbidity was renal transplantation, and 85% had deterioration of renal function. Five of the patients had undergone renal transplantation. The most common clinical manifestation was seizures. All had subcortical lesions and bilateral parietooccipital involvement was the finding most frequently observed.
ConclusionThis syndrome should be taken into account in the differential diagnoses of patients presenting with acute neurological syndromes and the abovementioned risk factors.
Describir las manifestaciones clínicas, antecedentes, comorbilidades y tratamientos asociados, hallazgos imagenológicos y seguimiento evolutivo de los pacientes con síndrome de leucoencefalopatía posterior reversible.
MétodosSe realizó un análisis retrospectivo y descriptivo de pacientes ingresados desde junio de 2009 hasta mayo de 2014, en un centro de tercer nivel de atención. Se evaluó edad, sexo, comorbilidades, sintomatología, valores de presión arterial al ingreso, función renal, medicación, tiempo transcurrido hasta la desaparición de síntomas.
ResultadosSe incluyeron 13 pacientes. El 77% estaba hipertenso al inicio del cuadro y el 85% presentó deterioro de la función renal. En 5 pacientes se objetivó el antecedente de trasplante renal. La manifestación clínica más común fueron convulsiones. Todos presentaron lesiones subcorticales y el compromiso más frecuente fue parietooccipital bilateral.
ConclusionesEste síndrome debe tenerse en cuenta entre los diagnósticos diferenciales de pacientes que se presenten con cuadros neurológicos agudos y los factores de riesgo mencionados.
Posterior reversible encephalopathy syndrome (PRES) is a potentially reversible clinical and radiologic entity which presents with acute neurological symptoms (disorders of consciousness, seizures, headache, visual disturbances, focal neurological deficit).1
It usually presents in the context of blood pressure fluctuations, renal failure (with or without dialysis), cytotoxic drugs, systemic autoimmune diseases (SAD) and preeclampsia or eclampsia.2
It may be caused by endothelial lesions relating to abrupt changes in blood pressure or direct effects of cytokines in the endothelium, with rupture of the hematoencephalic barrier and cerebral oedema.1–3
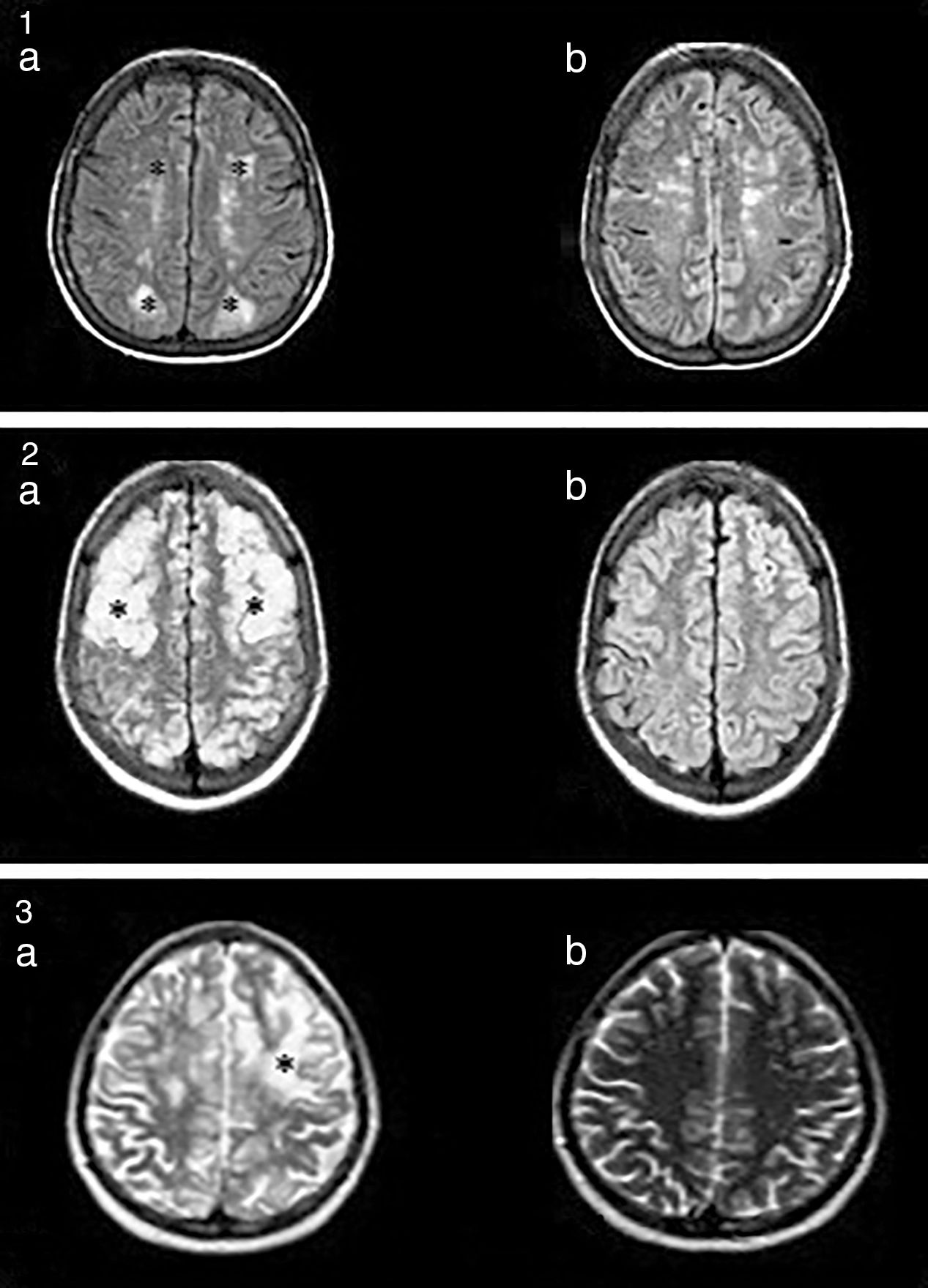
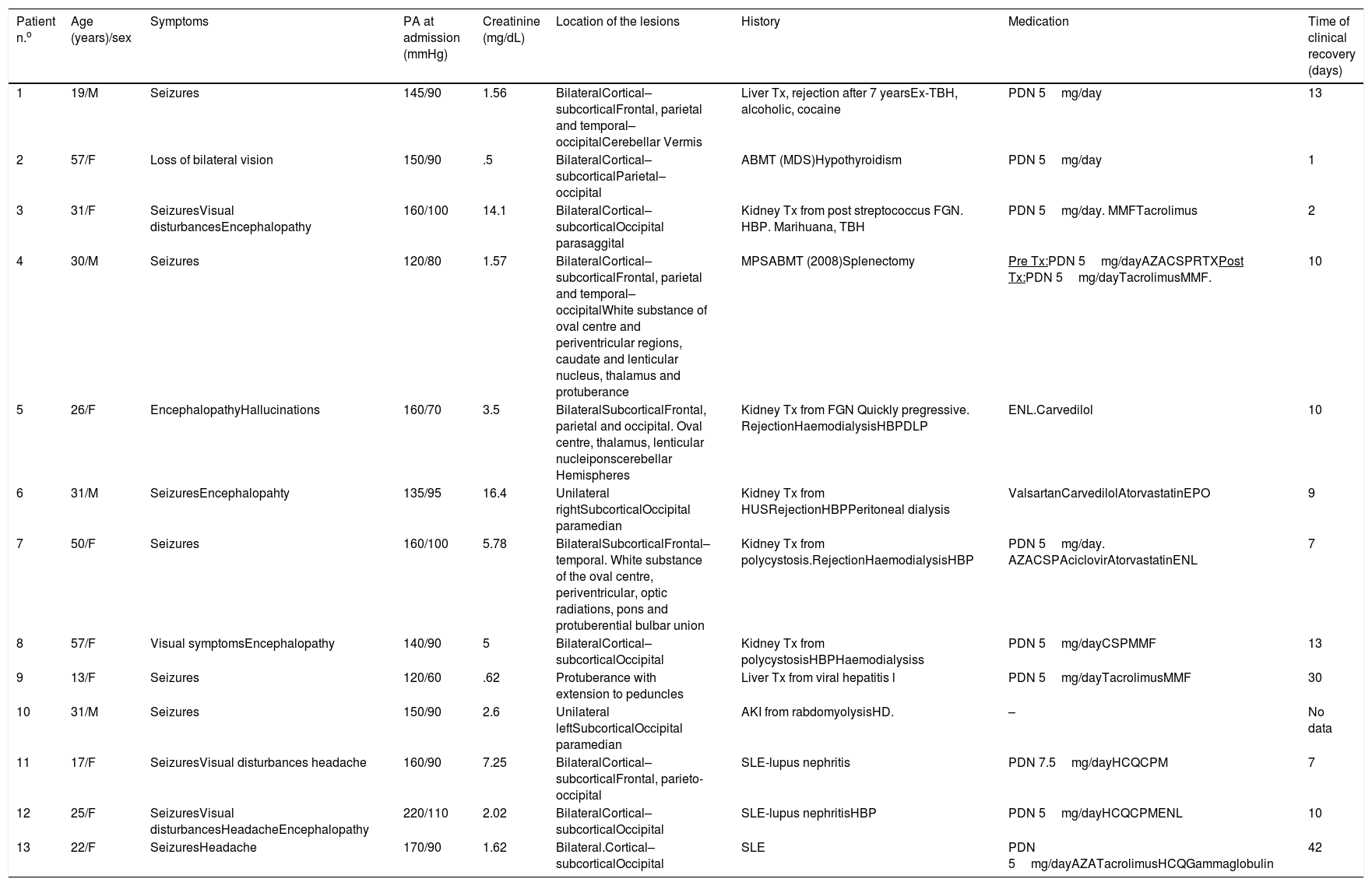
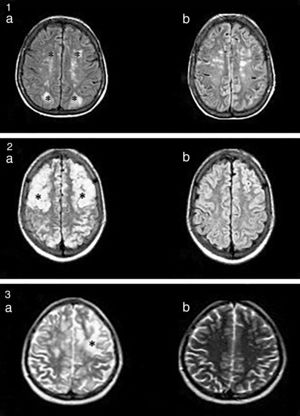
Nuclear magnetic resonance (NMR) (Fig. 1) is the method with the highest diagnostic value and differentiates this síndrome from acute ischaemic lesions.4
(1a) Hyperintense lesions on FLAIR, cortico-subcortical, symmetric, parieto-occipital (*). (b) Evidence of improvement after 3 months. (2a) Hyperintense images on FLAIR, well defined, bilateral, which compromise cortical–subcortical, frontal–parietal–temporal–occipital bilateral regions (*). (b) Proof of improvement after 2 months. (3) Hyperintense lesion in T2 (*). There were no signs of changes in the diffusion sequence.
This is a rare entity and its true prevalence is unknown. It is usually more common in women and there is no predilection for any specific age group.5 There are only a few available PRES reports.6,7
We present 13 cases exposing the form of presentation, antecedents and associated treatments, radiologic findings and disease evolution.
Patients and methodsA retrospective and descriptive analysis was performed on 13 patients who were admitted to hospital with a diagnosis of PRES from June 2009 to May 2014, in a third-level care hospital.
PRES was considered to exist in all patients with compatible radiologic symptoms and findings,1 assessed by the neurology department, excluding the most common differential diagnoses.1
Age, sex, medical history, symptoms, values of blood pressure at admission, renal function, medication, time elapsed since the disappearance of symptoms, imaging tests and lesion characteristics were assessed (Table 1), obtaining clinical history data from our hospital and from the admission records of the Rheumatology and Neurology services.
Clinical, analytical and radiologic findings.
| Patient n.o | Age (years)/sex | Symptoms | PA at admission (mmHg) | Creatinine (mg/dL) | Location of the lesions | History | Medication | Time of clinical recovery (days) |
|---|---|---|---|---|---|---|---|---|
| 1 | 19/M | Seizures | 145/90 | 1.56 | BilateralCortical–subcorticalFrontal, parietal and temporal–occipitalCerebellar Vermis | Liver Tx, rejection after 7 yearsEx-TBH, alcoholic, cocaine | PDN 5mg/day | 13 |
| 2 | 57/F | Loss of bilateral vision | 150/90 | .5 | BilateralCortical–subcorticalParietal–occipital | ABMT (MDS)Hypothyroidism | PDN 5mg/day | 1 |
| 3 | 31/F | SeizuresVisual disturbancesEncephalopathy | 160/100 | 14.1 | BilateralCortical–subcorticalOccipital parasaggital | Kidney Tx from post streptococcus FGN. HBP. Marihuana, TBH | PDN 5mg/day. MMFTacrolimus | 2 |
| 4 | 30/M | Seizures | 120/80 | 1.57 | BilateralCortical–subcorticalFrontal, parietal and temporal–occipitalWhite substance of oval centre and periventricular regions, caudate and lenticular nucleus, thalamus and protuberance | MPSABMT (2008)Splenectomy | Pre Tx:PDN 5mg/dayAZACSPRTXPost Tx:PDN 5mg/dayTacrolimusMMF. | 10 |
| 5 | 26/F | EncephalopathyHallucinations | 160/70 | 3.5 | BilateralSubcorticalFrontal, parietal and occipital. Oval centre, thalamus, lenticular nucleiponscerebellar Hemispheres | Kidney Tx from FGN Quickly pregressive. RejectionHaemodialysisHBPDLP | ENL.Carvedilol | 10 |
| 6 | 31/M | SeizuresEncephalopahty | 135/95 | 16.4 | Unilateral rightSubcorticalOccipital paramedian | Kidney Tx from HUSRejectionHBPPeritoneal dialysis | ValsartanCarvedilolAtorvastatinEPO | 9 |
| 7 | 50/F | Seizures | 160/100 | 5.78 | BilateralSubcorticalFrontal–temporal. White substance of the oval centre, periventricular, optic radiations, pons and protuberential bulbar union | Kidney Tx from polycystosis.RejectionHaemodialysisHBP | PDN 5mg/day. AZACSPAciclovirAtorvastatinENL | 7 |
| 8 | 57/F | Visual symptomsEncephalopathy | 140/90 | 5 | BilateralCortical–subcorticalOccipital | Kidney Tx from polycystosisHBPHaemodialysiss | PDN 5mg/dayCSPMMF | 13 |
| 9 | 13/F | Seizures | 120/60 | .62 | Protuberance with extension to peduncles | Liver Tx from viral hepatitis l | PDN 5mg/dayTacrolimusMMF | 30 |
| 10 | 31/M | Seizures | 150/90 | 2.6 | Unilateral leftSubcorticalOccipital paramedian | AKI from rabdomyolysisHD. | – | No data |
| 11 | 17/F | SeizuresVisual disturbances headache | 160/90 | 7.25 | BilateralCortical–subcorticalFrontal, parieto-occipital | SLE-lupus nephritis | PDN 7.5mg/dayHCQCPM | 7 |
| 12 | 25/F | SeizuresVisual disturbancesHeadacheEncephalopathy | 220/110 | 2.02 | BilateralCortical–subcorticalOccipital | SLE-lupus nephritisHBP | PDN 5mg/dayHCQCPMENL | 10 |
| 13 | 22/F | SeizuresHeadache | 170/90 | 1.62 | Bilateral.Cortical–subcorticalOccipital | SLE | PDN 5mg/dayAZATacrolimusHCQGammaglobulin | 42 |
AKI: acute kidney impairment; AZA: azatioprine; CPM: cyclophosphamide; CSP: cyclosporine; DLP: dyslipidemia; ENL: enalapril; HCQ: hydroxichloroquine; HD: haemodialysiss; HBP: high blood pressure; SLE:systemic lupus erythematous; MMF: mycrophenolate mofetil; PDN: prednisone; MDS: mylodyspastic syndrome; MPS: myeloproliferative syndrome; ABMT: autologous bone marrow transplant; TBH: tobacco habit; Tx: transplant.
Thirteen patients with PRES were identified (9 women and 4 men). The average age of presentation was 31.46 years (range: 13–57).
Associated comorbidities were higher blood pressure (HBP) in 6 patients (46%), and systemic lupus erythematous (SLE) in 3 (23%). All the patients with SLE presented with renal compromise, hypocomlementemia and positive anti-ADN. In 9 patients (69.2%) had already had a transplant: renal in 5 (39%) – 3 of them in dialysis due to rejection, hepatic in 2 (15%) and bone marrow in 2 (15%).
On admission, 10 patients (77%) presented with HBP (above 140/90mmHg). As well as the 4 patients in dialysis, another 7 suffered from renal failure, making a total of 11 patients (85%). Mean creatininemia was 4.8mg/dL (range from .5 to 16.4).
Regarding the previous or current use of immunosuppressants, 10 (77%) patients received oral steroids, 4 (31%) tacrolimus, 4 (31%) mycrophenolate mofetil, 3 (23%) azathioprine, 3 (23%) cyclosporine, 3 (23%) hydroxychloroquine, 2(15%) cyclophosphamide and one (8%) rituximab.
Regarding clinical presentation, 10 patients had seizures as their initial symptom (77%). Other signs were: visual disturbances (n=5; 39%), encephalopathy (n=5; 39%) and headache (n=3; 23%).
The average clinical recovery time was 13 days (range: 1–42 days). For one patient there was not sufficient data to estimate this period, but they presented with reversible symptoms.
Imaging studies were performed in all 13 patients.
11 (85%) patients underwent computerized axial tomography as an initial test. In 6 no lesions were found. All patients from this series had a NMR scan on admission and the following lesion distribution was revealed: in 10 (77%) patients in both brain hemispheres, in 2 (15%) unilateral lesions and in one (8%) there was only a compromise at brainstem level.
In 12 there were hemispheric lesions, all on a subcortical level (92%), with cortical damage in 8 of them (62%).
With regard to the compromised region, 11 had occipital lesions (84.6%), 5 parietal (39%), 5 frontal (39%) y 3 temporal (23%). Four patients had brainstem lesions (31%) and 2 cerebellum (23%). Gadolinum contrast NMR was only used in 3 patients to confirm extravasation of contrast to the parenchyma, because the majority presented with contraindications from severe impairment of renal function.
A control NMR was performed in 11 patients, with obvious improvement or disappearance of lesions in 8 cases (62%). In 8 this was performed prior to 30 days and with a minimum interval of 5 days from the admission NMR. The 3 patients with no changes in the control images did not present with any clinical worsening and the control NMR was performed within 7 days after the first NMR.
DiscussionPRES presents with acute neurological symptoms, among which encephalopathy has been reported (50%–80%), in addition to seizures (60%–75%), headache (50%), disturbances (33%), focal neurologic deficit (10%–15%) and status epilepticus (5%–15%).1 The principal clinical symptoms of our series were similar: seizures, followed by encephalopathy and disturbances.
It has been suggested that the sudden presentation of HBP or fluctuations in it would be the main factors involved, changing the self-regulation of blood flow to the brain and leading to endothelial dysfunction with increase of the perfusion and rupture of the haemoencephalic barrier.1,3,8,9 These phenomena would provoke extravasation of plasma and interstitial macromolecules.9 In our series, 77% of patients presented with HBP. Patients with PRES may have moderate to severe HBP, accelerated HBP and hypertensive encephalopathy.1 However, up to 15%–20% may be normal or hypotense, and the belief exists that there could be other factors involved.1
Endothelia dysfunction may also occur from direct effects of circulating cytokines (TNF alpha, interleukin 1, interferon gamma, raised expression of VEGF).3,8 As a result PRES has also been associated with many SAD including SLE, Sjogren syndrome, rheumatoid arthritis, scleroderma, vasculitis, and thrombotic thrombocytopenic purpura.6,10,11 In our report we only found patients with SLE, and similarly to other series, they all presented with seizures, headache, HBP and renal compromise.8 They also all had hypocomplementaemia and positive anti-ADN. Differential diagnosis should be performed in these patients with ischaemic or haemorrhagic cerebrovascular stroke, neuropsychiatric SLE, and infections of the central nervous system.8,10,11 During the first 48h after admission duplex supra-aortic trunk ultrasound, echocardiogram, electrocardiogram and cardiovascular monitoring were performed on both the patients with SLE and all other cases in this series. An ECG-Holter was performed in 5 patients. No sources of embolism or cardiovascular diseases were found in any of the patients to justify admission symptoms. An electroencephalogram was also performed to rule out status epilepticus in patients with seizures.
Some immunosuppressants used in the treatment of SAD are associated with the development of PRES (oral corticoids, microphenolate, cyclosporine, cyclophosphamide).1,6,12 In our series the majority had received oral steroids and over half had received at least one other immunosuppressant
Renal failure is a condition that presents in up to 50% of cases1 and was found in 85% of our patients.
With regard to the imaging diagnosis, computerized axial tomography without dye contrast reveal vasogenic oedema in some patients, but its use is highly limited.12–14 In 54% of our cases no lesions showed up on the computerized axial tomography which were obvious in the NMR. Characteristic changes in NMR are: hypointensity in T1, hiperintensity in T2, increase in sensitivity in FLAIR, and lack of restriction in the weighted diffusion sequences.12–14 These sequences enable differentiation of vasogenic odema to be made, which predominates in the white substance and leads to displacement of water to tissue level molecules, of the cytotoxic oedema, observed in grey substance and shows restriction in diffusion.12–15
The White subcortical substances is usually affected, but the cerebral cortex may be compromised.1–4,12–14 In all patients who presented with hemispheric lesions changes to the subcortical level were observed and in 62% there was associated cortical damage.
The posterior regions of the bran are particularly susceptible to the increase of perfusion and to HBP due to low sympathetic innervations.1 This would be explained by the fact that cerebral oedema usually more often affects the parietal-occipital regions.1–6 However, there are other frequent distribution pathways: the peripheral irrigation regions of both hemispheres and at superior frontal level.1,11–14 The involvement of the frontal and temporal lobule has been reported in up to a 75%.1 Compromise of the baseline lymph nodes, the cerebellum and brainstem, and even spinal cord have been described.1
In one patient we observed exclusive compromise of pons and peduncles. This rare location requires careful differential diagnosis with other syndromes affecting the brainstem.
Radiologic findings do not necessarily correlate with the severity of clinical features or prognosis.1,12
There are no specific treatments for this entity.12–14 HPB, blood gases and electrolyte control measures are suggested, together with anticonvulsants, withdrawal of potentially implicated drugs and the treatment of underlying disorders (sepsis, SAD, eclampsia, preeclampsia).1–7,12–14
In general prognosis is favourable and most patients recover both clinically and in imaging test controls.12–15 However, severe cases with irreversible lesions, intracranial or subarachnoid haemorrhaging and endocraneal hypertension have been published. Mortality is not above 6%.1,2,12–15
Current data derive from retrospective studies and case series. However, they have enabled the finding of atypical clinical presentations, radiologic findings in other topographies, rare complications, new drugs possibly implicated and different evolutionary pathways. Experimental studies are needed to advance knowledge on physiopathology and randomized trials, randomly distributed and multicentre to design guidelines and specific treatment objectives.
Although this entity is an uncommon complication, and probably also under-diagnosed in the SAD, we believe it is of great relevance to rheumatologists to determine knowledge of clinical characteristics, risk factors, diagnostic studies and therapeutic management. This is also based on the fact that in daily practice patients with SAD may develop this complication from the disease itself, from immunosuppressant treatments and renal failure and transplant.
ConclusionsIn our case series PRES presented in patients with a history of HBP, SLE and renal transplant. The seizures were the most common symptoms. Most patients presented with HBP at the time of diagnosis and they were being treated with immunosuppressants. NMR was the imaging method of choice.
Incorporating this entity as a differential diagnosis of acute neurological symptoms in patients with SAD may support an early diagnosis and be an appropriate treatment to prevent sequelae.
FinancingWe declare that we have not received any financing.
Conflict of interestsWe have no conflicts of interests to declare.
Please cite this article as: Pirola JP, Baenas DF, Haye Salinas MJ, Benzaquén NR, Colazo M, Borghi MV, et al. Síndrome de leucoencefalopatía posterior reversible: serie de casos y revisión de la literatura. Reumatol Clin. 2020;16:169–173.