Alendronate and risedronate are both effective and safe treatments for osteoporosis in men, but only risedronate has this indication in its data-sheet. We compared their use by gender.
Patients and methodsRetrospective descriptive study of prescriptions of risedronate and alendronate in 2012 in primary care in the northwest area of the Community of Madrid was conducted. We compared patients and defined daily doses (DDD) dispensed by gender.
Results14,857 patients used 1,847,370 DDD of alendronate or risedronate, 1145 (7.7%) patients were men. In women alendronate was most prescribed (55% vs. 45%) than risedronate. Risedronate was preferred in men, 47.6% vs. 52.4%, resulting in a statistically significant difference (p<.001).
ConclusionsRisedronate is preferred to alendronate in men, which is often used off-label, despite the existence of alternatives.
El ácido alendrónico y ácido risedrónico son eficaces y seguros para la osteoporosis del varón, pero solo el ácido risedrónico recoge esta indicación en su ficha técnica. Compararemos su uso en ambos sexos.
Pacientes y métodosEstudio descriptivo retrospectivo de prescripciones de ácido risedrónico y alendrónico en 2012 en atención primaria en el área noroeste de la Comunidad de Madrid. Comparamos la proporción de pacientes y dosis diarias definidas (DDD) dispensadas según género.
ResultadosCatorce mil ochocientos cincuenta y siete pacientes utilizaron 1.847.370 de DDD de alendronato y risedronato, 1.145 (7.7%) varones. En mujeres, alendronato fue un 10% más prescrito que risedronato (55% vs. 45%). En varones, el risedronato fue el preferido, 47.6% vs. 52.4%, con una diferencia estadísticamente significativa (p<0,001).
ConclusionesEl ácido risedrónico es preferido al alendrónico en varones, que se utiliza a menudo fuera de ficha técnica a pesar de existir alternativas.
Osteoporosis is the alteration of the structure and bone mineral density that produces bone frailty and susceptibility to fractures. Although it mostly affects older women with risk factors for fracture, prevalence according to densitometric criteria of the World Health Organization1 is important in Spanish men over 50 years of age: 4.8% for spinal osteoporosis and 4.4% for hip osteoporosis,2 five times lower than in women. As for established osteoporosis, the prevalence of vertebral fracture in men over 60 years is 21% (slightly less than women, 25%)2 and the incidence of hip fracture is 1.7 per 1000 inhabitants per year, a third compared to women.3
There are fairly safe and effective treatments used to improve bone mineral density in men at high risk, such as oral bisphosphonates, alendronic4, and risedronic acid5. Both have a similar efficacy and safety profile,6 and little difference in cost; however, while risedronic acid does have an indication for male osteoporosis in its data sheet7 alendronate only indicates it in women for “prevention of postmenopausal osteoporosis.”8
We intend to compare the gender distribution in dispensing alendronate, approved for the prevention of osteoporotic fractures in women but not in men in their technical insert versus risedronic acid, which has both indications.
Patients and MethodsWe carried out a retrospective descriptive study that collected all of the prescriptions for risedronic and alendronic acid (with or without vitamin D) in any oral form, made in 2012, which were given out by family physicians in the northwest of the Community of Madrid and dispensed in pharmacies. The data was obtained through the information system of the pharmaceutical services of the Community of Madrid (Farmadrid), which records dispensed medications. Data for defined daily dose (DDD) and different patients were compared by gender, excluding those who did not collect the prescription.
Qualitative variables are presented with their frequency distribution (absolute and relative). To establish whether there were significant differences in the selection of a drug, the χ method2 was used. The Z test for normal distribution was used to compare alendronate and risedronate usage rates by gender.
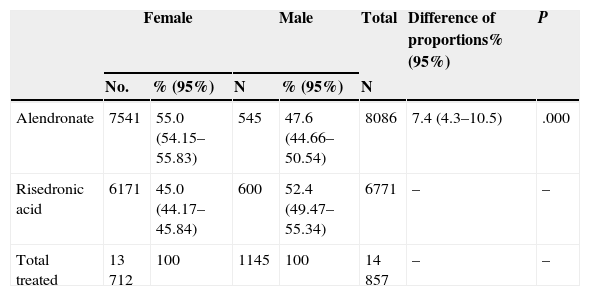
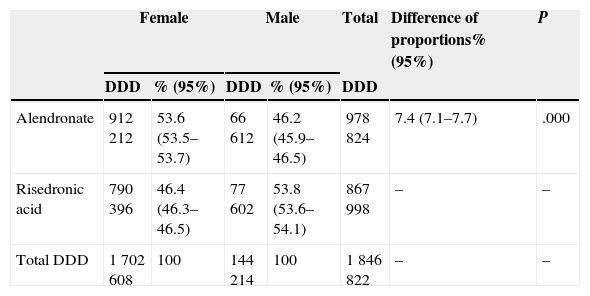
ResultsWe obtained data from 14,857 patients who used either of the two drugs, 1145 (7.7%) were men. 1 847 370 DDD were dispensed; alendronate was more prescribed with 979 104 doses (53%). 548 (0.03%) prescriptions were excluded for lack of a registered gender. Tables 1 and 2 report the gender distribution by number of patients and DDD.
Gender Distribution and Consumption of Risedronic and Alendronic Acid, in a Number of Patients.
| Female | Male | Total | Difference of proportions% (95%) | P | |||
|---|---|---|---|---|---|---|---|
| No. | % (95%) | N | % (95%) | N | |||
| Alendronate | 7541 | 55.0 (54.15–55.83) | 545 | 47.6 (44.66–50.54) | 8086 | 7.4 (4.3–10.5) | .000 |
| Risedronic acid | 6171 | 45.0 (44.17–45.84) | 600 | 52.4 (49.47–55.34) | 6771 | – | – |
| Total treated | 13 712 | 100 | 1145 | 100 | 14 857 | – | – |
Gender Distribution and Consumption of Risedronic and Alendronic Acid in DDD.
| Female | Male | Total | Difference of proportions% (95%) | P | |||
|---|---|---|---|---|---|---|---|
| DDD | % (95%) | DDD | % (95%) | DDD | |||
| Alendronate | 912 212 | 53.6 (53.5–53.7) | 66 612 | 46.2 (45.9–46.5) | 978 824 | 7.4 (7.1–7.7) | .000 |
| Risedronic acid | 790 396 | 46.4 (46.3–46.5) | 77 602 | 53.8 (53.6–54.1) | 867 998 | – | – |
| Total DDD | 1 702 608 | 100 | 144 214 | 100 | 1 846 822 | – | – |
DDD: defined daily dose.
55% of women and 47.6% of men were treated with alendronate; the difference in proportions was statistically significant (P<.001). The difference was also statistically significant (P<.001) compared to alendronate DDD in women (53.6%) and men (46.2%).
Women received 10% more alendronic than risedronic acid (55% versus 45%); on the contrary, among men, risedronic acid was prescribed 4.8% more than alendronic acid (52.4% versus 47.6%). These differences are similar when measured as DDD in favor of alendronate in women (53.6% versus 46.4%) and risedronate in men (53.8% versus 46.2%), with significant differences both for DDD (χ2=2913, P<.001) and for different patients χ2=23.31, p<0.001).
DiscussionDrugs do not include an indication in the data sheet when they really have no such indication because they are ineffective or unsafe, when their effectiveness is difficult to prove (rare diseases) or if, having been proven, have not received or are pending administrative approval for the indication.9 Alendronate has not obtained the indication for male osteoporosis in Spain, although it does have a certain degree of evidence in the prevention of vertebral fracture.4 Risedronic acid, with a similar efficacy,5,6 includes the indication in the insert.7
Some clinical guidelines include specific recommendations for the prevention of osteoporotic fracture in men, recommending both alendronate and risedronic acid with a grade D recommendation (based on expert opinion)10 and insisting that “drugs that have not been approved by regulatory agencies for osteoporosis in men should only be used if the authorized drugs cannot be used”.11 The European Drug Agency in 2007-4 allowed EU countries to include, in a presentation of 10mg daily alendronate (rarely used), an indication for male osteoporosis,12 but not in Spain, and not for the weekly 70mg dose.
We know that men receive less treatment for osteoporosis than women: six times less in Norway,13 12 times less in our region. We found few studies that assess what bisphosphonates to use; in a French observational study of 210 osteoporotic men receiving bisphosphonates, 65% took alendronate and 18% risedronate,14 reversing the observation of our study for the former, although the indication does not exist in France either.
Without addressing what the indications of antiresorptive drug therapy in men with fracture risk are, if the decision to install it is made, taking into account two options of similar efficacy, safety and price,6 the chosen drug must be the one with the indication expressly written in the data sheet. Not because it is prohibited, it is not forbidden to prescribe off-label, but because the enabling conditions to use them are not verified. One might therefore expect infrequent use of alendronate in men; however, doctors often have opted for it. Our study detected a preference in favor of risedronate in men versus alendronate, unlike women; although statistically significant, the difference is small in magnitude, 5%–10%. These differences persist if we compare the number of different patients or number of doses received, indicating that the doses received were similar in both genders.
The use of drugs outside the approved indication in the data sheet is possible, even common, in specialties such as oncology, for compassionate use, but uncommon in primary care.9 The National Health System regulates it by the Royal Decree 1015/2009 of 19 June, with three modes: compassionate use of investigational drugs, not marketing drug use in Spain and use of drugs in different conditions than authorized. In the latter case “conditions for prescribing approved medications when used other than authorized, which in any case will be considered as exceptional conditions” are set, such as the absence of authorized therapeutic alternatives, with respect to restrictions on prescribing and dispensing, following a therapeutic protocol of the clinic, exceptional and justified use expressed in the medical history by the prescribing physician, and ensuring the reporting of adverse reactions detected. Furthermore, information and the patient informed consent must be obtained, in accordance with Law 41/2002 of Patient Autonomy.
The use of alendronate in men for osteoporosis does not meet all requirements; it is not exceptional, as we see, there are alternatives6 and there is no therapeutic protocol, at least in the study centers. Our research did not examine whether the justification was contained in the medical history, or if patient consent was reported for off-label use obtained; however, from our experience, we doubt that these requirements were met.
Why is there such a widespread use of alendronate in men? Our study does not allow causal hypotheses. We attribute, as possibilities, ignorance by the physician of off-label indications and prescription induced from hospital care, inadequate promotion by the pharmaceutical industry, the lack of drug alerts or that state and regional drug agencies assessment give priority of drug use among its recommendations to alendronate compared with other bisphosphonates, although a thorough reading shows that its conclusions are focused on postmenopausal women.15
Nor is it justified for the treatment of corticosteroid osteoporosis, where risedronic acid has the indication in the data sheet, unlike alendronic acid.7,8
The use of drugs outside the licensed indication generates no direct legal consequences for the sake of the prescription, provided they do not harm the patient. However, if damage occurs, even a mild and expected side effect (for example, esophagitis), the injured party may demand accountability from the prescriber to repair the damage caused, as has already happened (Case Court Litigation 1 N. Sevilla, from February 6, 2006)16; the injured argued that the physician did not follow the conditions regulated for off-label prescribing, including lack of patient information, which created responsibility for the prescriber and the institution.
While no indication for use in males is approved as an indication in the data sheet, alendronate should be reserved for older women with risk factors for fracture. There are alternatives like risedronic acid treatment when it is indicated, which is also effective and has a similar safety profile and posology. Only in rare cases should alendronate be used in men, duly informing the patient that the prescription is done off-label in the data sheet.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare this research did not perform experiments on humans or animals.
Confidentiality of dataThe authors state that no patient data appears in this article.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
Conflict of InterestFernando León Vazquez has imparted courses sponsored by MSD. There are no other conflicts of interests.
Please cite this article as: León Vázquez F, Herrero Hernández S, Cuerpo Triguero C, Andrés Prado MJ, Cabello Ballesteros L. Prescripción de ácidos alendrónico y risedrónico en varones: uso fuera de la ficha técnica en un área de salud. Reumatol Clin. 2015;11:64–67.