Sjögren's syndrome (SS) is a systemic autoimmune disease that mainly affects the exocrine glands. It has been reported that the association between SS and respiratory involvement occurs in 11% and 45% of cases, and can be the initial manifestation of the disease. The diagnosis is based on the integration of clinical, immunological and histopathological criteria. This paper examines a 58-year-old man with SS, severe dyspnea and recurrent exacerbations associated with bronchiolitis–bronchiectasis. The absence of anti-Ro and anti-La antibodies associated with severe bronchiolitis is a clinical presentation that is poorly characterized in this group of patients; moreover, how to treat them remains unclear.
El síndrome de Sjögren (SS) es una enfermedad sistémica autoinmune que afecta principalmente a las glándulas exocrinas. Se ha reportado que la asociación entre SS y compromiso respiratorio ocurre entre el 11 y el 45% de los casos. El diagnóstico se basa en la integración de criterios clínicos, inmunológicos e histopatológicos. Se presenta el caso de un masculino de 58 años, con SS, disnea grave y exacerbaciones recurrentes de la vía respiratoria, asociado a bronquiolitis-bronquiectasias. La ausencia de anticuerpos anti-Ro y anti-La, relacionados con la gravedad de la bronquiolitis, es una forma de presentación poco caracterizada en este grupo de pacientes, más aún, su tratamiento óptimo no está establecido.
Sjögren's syndrome (SS) is an autoimmune disease that mainly affects the exocrine glands; however, it can involve extraglandular organs.1–3 Anti-Ro antibodies are negative in 5–10% of the patients and salivary gland biopsy is indicative of the diagnosis in 69% of the cases.3 Interaction between SS and respiratory involvement occurs in up to 45%; the most common complications include bronchiectasis and interstitial lung disease.4–6 However, there are few reports that describe the clinical profile of SS and bronchiolitis.7 We present the case of a man with progressive dyspnea with seronegative primary SS associated with bronchiolitis.
Clinical ObservationThe patient was a 58-year-old man. The Coombs test was negative. He did not smoke and had not been exposed in or out of the working environment to organic or inorganic substances or fungi and did not have gastroesophageal reflux disease. He had a 5-month history of symptoms characterized by burning eyes, coughing fits and scanty mucohyaline expectoration. He mentioned having recurrent episodes defined as respiratory tract infections. Physical examination revealed conjunctival hyperemia; fine crackles and rhonchi were detected in subaxillary and infrascapular regions in both hemithoraces. There were no other abnormalities in the rest of the examination.
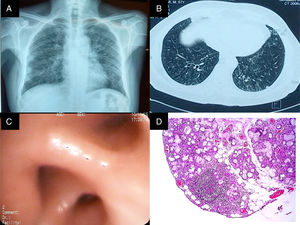
Chest radiography (Fig. 1 panel A) showed opacities that involved the lung interstitium. Thus, the patient underwent high-resolution computed tomography of the chest that revealed the presence of bronchiectasis, centrilobular nodules and areas illustrating the tree in bud pattern (Fig. 1 panel B). The analysis of the pulmonary function revealed severe restriction without reversibility (Table 1). On the basis of the findings recorded, we requested that he undergo bronchoscopy, which showed edema of the bronchial mucosa (Fig. 1 panel C); the pathological analysis of the biopsy only demonstrated neutrophil infiltration. On the other hand, the culture of the specimen and bronchoalveolar lavage (BAL) for acid-alcohol fast bacilli and bacteria, and culture in Sabouraud agar were negative. In addition, C-reactive protein in the biopsy and BAL for Mycobacterium tuberculosis were also negative, as were the rheumatology panel and other pertinent paraclinical panels (Table 2).
(A) Posterior–anterior chest radiograph. Images compatible with bilateral reticulonodular pattern, predominantly basal, with conserved lung size. (B) High-resolution computed tomography of the chest with images of the signet ring in both lungs, centrilobular nodules and budding tree pattern. (C) Bronchus of the right lower lobe with edema and concentric narrowing of the orifices of the lung base. (D) Biopsy of the salivary gland. Predominantly lymphocytic inflammatory infiltrate, with the formation of 2 lymphoid aggregates of more than 50cells/mm2.
Analysis of Pulmonary Function in the During Follow-up of Our Patient.
| Variable | Baseline (January 2016) | Follow-up 1 (May 2016) | Follow-up 2 (August 2016) | Delta follow-up 1-baseline | Delta follow-up 2-baseline |
|---|---|---|---|---|---|
| Post-bronchodilator spirometry | |||||
| FVC (l) | 1.46 | 1.44 | 1.71 | −0.02 | 0.25 |
| FVC (%p) | 41 | 40 | 47 | Absolute: 1%p | Absolute: 6%p |
| Real: −1.3%p | Real: 17.1%p | ||||
| FEV1 (l) | 1.13 | 1.25 | 1.42 | 0.12 | 0.29 |
| FEV1 (%p) | 42 | 45 | 52 | Absolute: 3%p | Absolute: 10%p |
| Real: 10.6%p | Real: 25.6%p | ||||
| FEV1/FVC | 78 | 86 | 83 | 8 | 5 |
| Arterial blood gases | |||||
| pH | 7.49 | N/R | 7.45 | −4 | |
| PaCO2 (mmHg) | 32 | N/R | 33 | 1 | |
| PaO2 (mmHg) | 56 | N/R | 61 | 5 | |
| HCO3− mmol/L | 24.4 | N/R | 24 | −0.4 | |
| Lactate mmol/L | 1.3 | N/R | 1 | −0.3 | |
| A-a O2 (mmHg) | 53.73 | N/R | 47.48 | −6.25 | |
| SpO2 (%) | 90 | N/R | 91 | 1 | |
| 6-minute walk | |||||
| Meters | N/R | 484 | 492 | 8m | |
| Final dyspnea | N/R | Borg 3 | Borg 1 | −2 units | |
| Final fatigue | N/R | Borg 2 | Borg 1 | −1 unit | |
A-a O2, alveolar-arterial oxygen gradient; absolute delta, recorded difference based on the change in the units in terms of percentages taking the baseline as a reference; real delta, recorded difference based on the change in lung volume taking the baseline as a reference; FEV1, forced expiratory volume in the first second; FEV1/FVC, FEV1/FVC ratio; FVC, forced vital capacity; HCO3, bicarbonate; N/R, not reported; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; SpO2, peripheral oxygen saturation by pulse oximetry; %p, percentage of predicted value.
Findings in Immunological Studies in Serum.
| Laboratory test | Result | Normal range |
|---|---|---|
| C-reactive protein | 37mg/dL | 0–5 |
| Erythrocyte sedimentation rate | 37mm/h | 0–20 |
| Rheumatoid factor | Negative | Positive |
| Anti-native DNA | 0.70IU | 0–0.90 |
| Anti-SSA/Ro (IgG) | 0.30IU | 0–0.9 |
| Anti-SSB/La (IgG) | 0.38IU | 0–0.90 |
| Anti-CCP | 6.5U/mL | 0–9.9 |
| Anti Scl-70 | Negative | Highest titer 1:40 |
| Alpha-1-antitrypsin | 257mg/dL | 88–174 |
| Chlorides in sweat | 50mmol/L | >80 |
| IgG, IgE, IgM, IgA, IgD | Normal ranges |
Ig, immunoglobulin; anti-CCP, anti-cyclic citrullinated peptide antibodies; IU, international units.
In view of the history of conjunctival hyperemia, the patient underwent the Schirmer test, which was positive. Given this finding, and the negative results in the tests for antibodies, we performed a biopsy of the salivary gland that revealed chronic sialoadenitis grade 4.1 in the Chisholm-Mason classification (Fig. 1 panel D). Currently, the patient is taking steroids, methotrexate, bronchodilators, macrolides and inhaled corticosteroids, that results in less severe symptoms and an improvement in pulmonary function (Table 1).
DiscussionThe present case is relevant for 2 reasons: (1) the fact that SS was seronegative and respiratory manifestations were infrequent and (2) the association of bronchiolitis with manifestations of a severe deterioration of lung function in SS.
On the basis of the European League Against Rheumatism (EULAR) SS disease activity index (ESSDAI), the lung is the second most frequently involved organ in these clinical conditions.6 It was considered that interstitial lung disease was the main manifestation affecting the respiratory tract; however, the incidence of bronchiectasis and/or bronchiolitis has been found to be increasingly higher.8 We wish to point out that anti-Ro/SSA antibodies are detected in 27–50% of the patients with SS and bronchiectasis; of these, only 41% fulfill the diagnostic criteria of the American and European consensus.9 Moreover, the scenario changes if we utilize the criteria of the Sjögren's International Alliance, given that only 27% of the patients meet the diagnostic criteria.3 Therefore, we can reflect on that the possibility that serological markers should not be considered, per se, as diagnostic indicators as there are patients in whom these antibodies are negative. The clinical expression of the disease obliged us to rule out, using a route of greater to lesser frequency, the causes that could be related to respiratory findings in our patient. On the basis of this argument, upon systematically excluding the most common causes of bronchiolitis (for example, tuberculosis, mycosis, exposure to inorganic substances, etc.), we focus on the ocular manifestations involving sicca syndrome as a key datum to perform a biopsy of the salivary gland. On the other hand, the centrilobular nodules and budding tree pattern documented in the present case are usually related to follicular or lymphocytic bronchiolitis, and these tomographic findings have been reported in 6–24% of patients with SS and lung involvement.2,4
As far as we know, changes in SS that come to affect the small airways have not been reported in the Latin population and their behavior in another population is rarely associated with severe symptoms.7 Likewise, it is infrequent to consider a diagnosis of SS as a differential factor in scenarios of chronic bronchiolitis.4,7,9
There are reports that point out that pulmonary function tests in patients with SS and respiratory involvement usually reveal patterns of mild restriction or obstruction.4,9,10 Nevertheless, Borie et al. have reported cases similar to ours, in which there was greater involvement of the pulmonary function characterized by more severe restrction.7 A limitation for understanding this form of presentation is that, given that there is no clear characterization, the efficacy of certain treatments is unknown.4
ConclusionsThe evidence of involvement of small airways with serious functional impairment and recurrence of bronchial exacerbations makes it necessary to rule out alternate scenarios including autoimmune diseases like SS. The association between clinical and histological symptoms is key in the diagnosis despite a negative serology.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
The authors with to thank electronic engineer Baltazar Cortés-Tellez for his help in editing the images.
Please cite this article as: Medina-Paz L, Che-Morales JL, Barrera-Pérez HAM, Cortes-Telles A. Síndrome de Sjögren primario y bronquiolitis. Una asociación no usual. Reumatol Clin. 2018;14:233–235.