Despite screening for latent tuberculosis (TB), new cases of TB infection are detected in patients treated with anti-TNF-α and negative initial screening, some of them after long treatment, which points more to a new infection.
ObjectivesTo describe the cases that have presumably developed a primary tuberculous infection during treatment with anti-TNF-α drugs.
MethodsRetrospective audit (1999–2012). Inclusion criteria were: (a) anti-TNF-α treatment; (b) initial latent TB screening negative; (c) TB diagnosed during anti-TNF-α treatment; (d) suspected primary TB infection (diagnosis after at least 12 months on anti-TNF-α). Clinical, epidemiological, therapeutic and outcome variables were reviewed.
ResultsTwo cases of primary TB infection were found out of 771 anti-TNF-α treated patients (0.2%). One woman aged 41 suffered TB pneumonia after 35 months of treatment with adalimumab, and a male aged 37 who developed disseminated TB after 107 months of treatment with infliximab.
ConclusionsAlthough uncommon, during TNF antagonist therapy, TB risk persists despite negative initial screening, so clinicians should be aware of TB during the entire treatment.
A pesar de las medidas de cribado de tuberculosis (TB) siguen detectándose casos en pacientes tratados con anti-TNF-α y cribado inicial negativo, algunos tras largo tiempo de tratamiento, lo que apunta más a una nueva infección.
ObjetivosDescribir los casos que presumiblemente han desarrollado primoinfección tuberculosa durante el tratamiento con fármacos anti-TNF-α.
MétodosRevisión retrospectiva (1999-2012), seleccionando según los siguientes criterios: a) tratamiento anti-TNF-α; b) cribado de TB inicial negativo; c) TB diagnosticada durante tratamiento anti-TNF-α, y d) sospecha de primoinfección tuberculosa (tras mínimo 12 meses de anti-TNF-α). Se han revisado sus variables clínicas, epidemiológicas, terapéuticas y de desenlace.
ResultadosDos casos de primoinfección tuberculosa de 771 pacientes tratados con anti-TNF-α (0,2%). Una mujer de 41 años y 35 meses de tratamiento con adalimumab y un varón de 37 años y 107 meses de tratamiento con infliximab. La mujer presentó una neumonía y el varón una TB diseminada.
ConclusionesDurante la terapia anti-TNF-α persiste el riesgo de TB a pesar de cribado inicial negativo, por lo que el grado de sospecha debe ser elevado durante todo el tratamiento.
Biologic therapies with tumor necrosis factor-alpha antagonists (anti-TNF-α) have revolutionized the prognosis of rheumatic diseases. Their use, however, is accompanied by certain risks. One of the adverse effects to be taken into account is the increase in the number of infections,1 especially of the opportunistic and granulomatous types, such as tuberculosis (TB). Although the risk is increased with all the anti-TNF-α agents, there are data that suggest a higher risk among patients who are treated with monoclonal antibodies when compared with those receiving the fusion protein.2
Since 2002, the United States Food and Drug Administration recommends screening for TB, and chemoprophylaxis in case of latent TB infection, before initiating anti-TNF-α agents. Prophylaxis is recommended in the following situations3: (a) recent exposure to confirmed TB; (b) a history of TB without proper treatment; and (c) positive Mantoux test and/or radiographic evidence of sequelae of TB. This measure has dramatically reduced the incidence of TB among patients receiving anti-TNF-α4; however, cases of TB continue to be detected, despite an initial negative screening test.5 This could be due to either a reactivation of latent TB with a negative screening test (false negative) or a primary tuberculous infection. The time to development of active TB after initiation of anti-TNF-α therapy may prove to be the clue to whether reactivation or primary infection should be suspected, as reactivation can be expected to occur within a short time after initiation of immunosuppressive therapy. However, a tuberculous infection after a long period of treatment suggests a primary infection, although it has not yet been possible to establish a clear time limit to distinguish between the 2 origins.6
Given its relevance, the objective of this study is to review the cases of active TB in which the patients presumably developed a primary tuberculous infection during treatment with anti-TNF-α agents, despite an initial negative screening test.
Material and MethodsWe performed a retrospective review of the registry of patients receiving biologic therapy in the rheumatology department of Hospital General Universitaria de Alicante, in southeastern Spain, which included a total of 771 patients treated between 1999 and 2012 (year of the study). We selected the medical records of patients who met the following inclusion criteria: (a) current or previous treatment with anti-TNF-α; (b) negative results in TB screening prior to treatment with anti-TNF-α; (c) active TB diagnosed during treatment with anti-TNF-α; and (d) suspected primary tuberculous infection; for this study, primary tuberculous infection was considered if the diagnosis had been made after treatment with the biologic agents for at least 12 months.7 The TB screening protocol in our center consists of the Mantoux test (1 or 2 steps), chest radiography, and microscopic study of sputum and urine specimens, when appropriate. We reviewed the clinical variables (signs of underlying disease and of tuberculosis), epidemiological variables (treatment duration, study of contacts), therapeutic variables (treatments received and duration) and outcome variables (test results and response to treatment) of the patients who met the inclusion criteria.
ResultsOf the 771 patients included in the registry, 7 had active TB. One had not undergone screening for TB prior to initiation of anti-TNF-α therapy, as this took place before the recommendations. The results of the Mantoux test were positive in 2; 1 of them received complete chemical prophylaxis (a short 3-month regimen of rifampicin and isoniazid) and the other, incomplete. In the remaining 4 patients, screening (including 2-step Mantoux test and chest radiography) was negative. Two of them developed TB during the first year of anti-TNF-α therapy and, therefore, were considered to have a reactivation of latent TB, with false negative results in the screening test. Finally, 2 patients (0.2% of the series) met all the inclusion criteria, and were considered to have primary tuberculous infections.
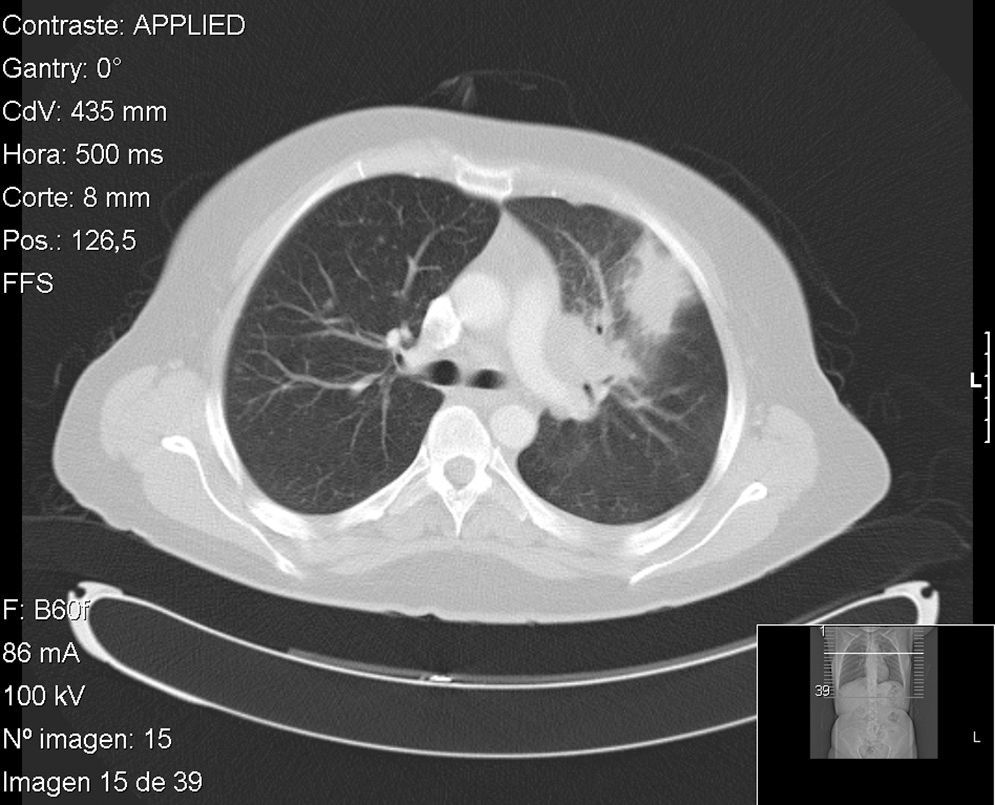
Case no. 1: The patient was a 41-year-old HLA-B27-positive woman diagnosed, at the age of 30 years, as having ankylosing spondylitis affecting the axial skeleton and recurrent acute anterior uveitis. Her response to nonsteroid anti-inflammatory drugs (NSAID) was suboptimal and treatment with adalimumab was begun in 2006, following screening for TB (at the time of the Mantoux test, she was only being treated with NSAID). In 2009, after 35 months of adalimumab, she presented with fever, dry cough, odynophagia, and headache, and was feeling generally unwell. Pulmonary computed tomography revealed a mass measuring 4cm in the anterior segment of left upper lobe, with suprahilar and prevascular adenopathy (Fig. 1). On this occasion, she reacted to the Mantoux test with an induration measuring 20mm 48h later. Fiberoptic bronchoscopy was performed with transbronchial biopsy and the study of the specimen revealed a mucosa with chronic granulomatous inflammation plus necrosis, and the culture was positive for Mycobacterium tuberculosis. After tuberculostatic therapy with rifampicin, pyrazinamide and isoniazid, the patient's disease resolved.
In the study of her contacts, her father proved to be positive for latent tuberculous infection, but no infectious contacts were found. Five months after completing the tuberculostatic therapy, the inflammatory low back pain returned and, after 2 episodes of acute anterior uveitis, the decision was made to reinitiate treatment with anti-TNF-α, in this case with etanercept; there were no incidences.
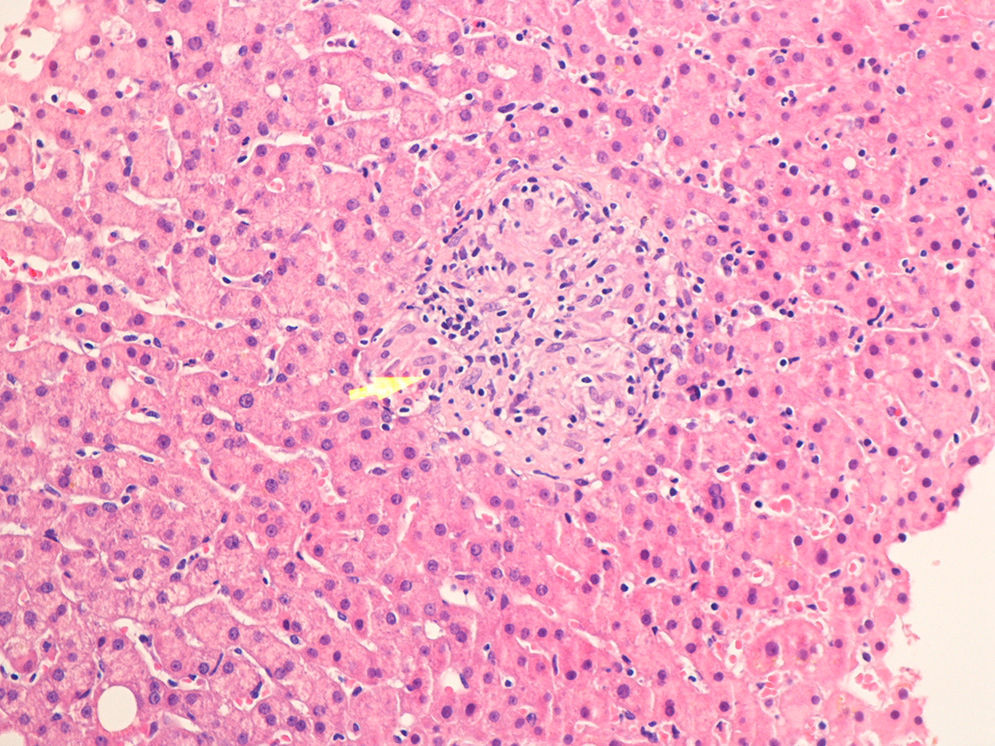
Case no. 2: This patient was a 37-year-old HLA-B27-positive man who had been diagnosed at the age of 14 years as having ankylosing spondylitis, with axial and peripheral involvement. Initially, he had been treated with sulfasalazine and NSAID, but in 2003, monotherapy with infliximab was begun because of the persistence of disease activity. Prior to the change in treatment, the patient underwent screening for TB while he was still being treated with sulfasalazine and NSAID. In 2012, after 107 months of treatment with infliximab, he developed a cough with expectoration, followed by dyspnea, fever and constitutional symptoms. Plain radiography revealed a suprahilar infiltrate in right lung and pulmonary computed tomography confirmed the presence of an alveolar infiltrate in segment 3 of right lung, with presence of adjacent hilar adenopathy. The Mantoux test was repeated, which this time was positive (17mm), and M. tuberculosis was isolated in both sputum and in the bronchial biopsy. After 2 weeks of tuberculostatic therapy with isoniazid, pyrazinamide, rifampicin and ethambutol, an increase in the cholestatic enzymes was detected, and ultrasound revealed the presence of hepatomegaly and there were multiple hypoechoic images in spleen. A liver biopsy confirmed the presence of tuberculous infection (Fig. 2). Thus, the definitive diagnosis was disseminated TB with pulmonary, hepatic and splenic involvement.
The study of the patient's contacts revealed no other cases of active TB, but latent tuberculous infection was detected in 2 relatives of advanced age.
The patient completed 7 months of treatment, with complete resolution of the disease, although during the tuberculostatic therapy, he developed atelectasis in right upper lobe due to endobronchial granuloma, which was attributed to an immune reconstitution phenomenon. Six months after the initiation of tuberculostatic therapy, due to a marked inflammatory activity of his ankylosing spondylitis, the decision was made to reinitiate anti-TNF-α therapy with etanercept. There were no further TB-related incidents, and monitoring of the activity of the inflammatory disease continued.
DiscussionTumor necrosis factor α is one of the essential cytokines in the formation and maintenance of the granulomatous inflammatory response that is capable of controlling M. tuberculosis.8 Thus, its inhibition increases the susceptibility to developing this infection. The inclusion in routine clinical practice of screening techniques for latent TB prior to initiating anti-TNF-α therapy has resulted in a decrease in the number of cases of active TB secondary to the use of these agents.4 However, the risk of developing TB remains high in these patients throughout the entire treatment period. In our series, we detected 2 cases involving patients with initial negative screening who developed active TB after 35 and 107 months of treatment with anti-TNF-α. It seems reasonable to assume that these were cases of primary tuberculous infection, since the disease appeared after a long period of immunosuppression. We used 12 months as the lower limit at which a case can be considered new TB infection. This date is arbitrary and is debatable, but in a series 703 patients treated with anti-TNF-α in the United Kingdom, all the reactivations occurred within the first year of treatment. It is also supported by the fact that, using a mathematical model, Wallis6 estimated that, after one year of treatment with infliximab, the predominant cause of TB would be primary infection. In any case, we cannot rule out the possibility that cases of TB detected during the first year of treatment could also be due to new infection.
The persistence of the risk of TB during treatment with anti-TNF-α leads to the question as to whether TB screening should be repeated periodically.8 Probably, by analogy with a proposal issued by the United States Centers for Disease Control and Prevention in Atlanta, Georgia, for patients with human immunodeficiency virus infection,9 the possibility of screening for TB each year throughout the duration of anti-TNF-α therapy has been considered; however, this strategy has not been formally tested in rheumatic patients and raises certain doubts, especially if there is no clinical suspicion and has been no possible contact with an infectious patient (positive Combe sign). The goal is the detection of latent tuberculous infection; however, should these patients contract the disease, they would probably have active TB, as in the cases reported here, because of the deficient granulomatous inflammatory response secondary to the biologic therapy. Another point that should be taken into account is a possible state of immunological anergy when a patient is undergoing the Mantoux test, since factors such as immunosuppression (especially corticosteroids) and age could result in false negatives.10
In short, we feel it necessary to stress the fact that the risk of developing TB persists in patients being treated with anti-TNF-α, even when initial screening was negative. Despite its low frequency in our series, the associated rates of morbidity and mortality indicate that a high suspicion should be maintained throughout the entire period of treatment with anti-TNF-α.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
We thank Dr. Miguel Trigueros, of the pathology department, for the images of the biopsy.
Please cite this article as: Bernal JA, Andrés M, Jovaní V, García Sevila R, Begazo A, Vela P. Primoinfección tuberculosa en pacientes con anti-TNF-α y cribado inicial negativo. Reumatol Clin. 2016;12:81–84.