Behçet's disease is a systemic vasculitis characterized by the presence of oral and genital ulcers. Neurological involvement or neuro-Behçet is an uncommon manifestation. It manifestation has predominance in the male gender appearing 2–4 years after the first clinical manifestation.
However, neuro-Behçet disease sometimes occurs with pseudotumoral brain lesions. Herein, we present the cases of two patients diagnosed with neuro-Behçet after detection of pseudotumoral brain lesions. A review of the literature is performed.
La enfermedad de Behçet es una vasculitis caracterizada por úlceras bucales y genitales. La afectación neurológica o neuro-Behçet es una manifestación infrecuente, de predominio en el género masculino y que aparece de 2 a 4 años después de la primera manifestación clínica.
El neuro-Behçet cursa ocasionalmente lesiones cerebrales pseudotumorales. Presentamos 2 casos de pacientes diagnosticados de neuro-Behçet tras la detección de lesiones cerebrales pseudotumorales y se realiza una revisión de la literatura.
Behçet's disease (BD) is a recurrent systemic vasculitis that is diagnosed on the basis of clinical criteria.1 Neurological involvement, although uncommon, is among the causes of higher morbidity and mortality rates, and is included in the differential diagnosis of inflammatory and demyelinating diseases of the central nervous system.2 The prevalence of neuro-BD (NBD) ranges between 1% and 59%, according to different series,1–5 and it is more common among men. Neurological involvement frequently develops 5 years after the diagnosis of BD,2 but may be a form of onset, which masks the diagnosis because of its atypical presentation.1,3 Parenchymal or extraparenchymal involvement can be observed and, in rare cases, it has been reported to present in the form of a pseudotumor.2,4,6–14
We present the cases of 2 patients who were diagnosed with NBD when pseudotumors of the brain were detected.
Case reportsCase no. 1The patient was a 63-year-old man who presented with a 1-month history of behavioral changes in the form of irritability, aggressiveness and emotional lability, as well as difficulty in walking and agraphia. He mentioned a 35-year history of recurrent oral ulcers, and that sores had appeared on his genitals over the preceding 6 months, accompanied by folliculitis in the genital region and lower extremities. In the physical examination, he was found to have temporospatial disorientation; bradypsychia; right facial, brachial and femoral paresis (4/5); and tendency to list or veer toward the right when walking; together with oral and genital ulcers. The most noteworthy laboratory findings were elevated acute phase reactant levels, negative results in serological tests for Brucella, syphilis, hepatitis B virus, hepatitis C virus and human immunodeficiency virus, and negative findings in an autoimmunity study. He tested positive for HLA B51. Brain magnetic resonance imaging (MRI) revealed the presence of a space-occupying lesion in left thalamus (Fig. 1.1). An angiographic study of the supra-aortic trunks revealed small-vessel disease and atheromatous lesions in the carotid bifurcations, as well as saccular aneurysms and infundibula, with images of vascular nidi in the left carotid territory suggestive of vasculitis. Thoracoabdominal computed tomography was normal. Lumbar puncture yielded clear cerebrospinal fluid, with an unremarkable protein content, without oligoclonal bands. As NBD was suspected, treatment was initiated with methylprednisolone pulse therapy, followed by oral prednisone (1mg/kg body weight [bw]/day), colchicine (1mg/12h) and oral azathioprine (2mg/kg bw/day). The patient's clinical status improved, his genital ulcers disappeared and there was no evidence of the neurological manifestations. After 3 months of treatment, he underwent follow-up brain MRI which revealed the complete resolution of the lesion (Fig.1.2). After 15 years of follow-up, the patient has no clinical neurological symptoms and is receiving absolutely no treatment. However, he has had sporadic oral aphthae, which have responded well to colchicine.
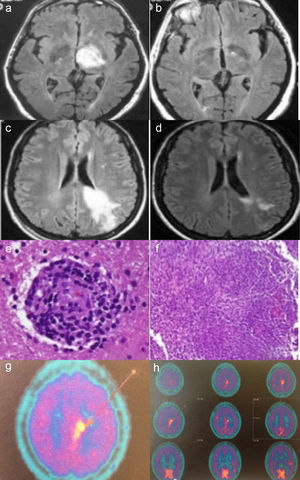
Case no. 1: Axial fluid-attenuated inversion recovery (FLAIR) magnetic resonance image (MRI) shows a hyperintense lesion in left thalamus (a). In an image acquired 2 months later, complete resolution of the lesion is observed (b). Case no. 2: Axial FLAIR MRI shows a hyperintense lesion in white matter of the left frontoparietal region, extending to the cortex and corpus callosum, in a gadolinium-enhanced image (c). MRI performed after 20 days of corticosteroid therapy shows a significant reduction in the hyperintense area (d). Histopathological findings in brain biopsy show a perivascular lymphocytic infiltrate with blood vessel involvement (hematoxylin-eosin) (e). Granulomatous inflammatory infiltrate (hematoxylin-eosin) (f). Brain 11C-methionine positron emission tomography (PET) images showing parasagittal deposition of 11C-methionine suggestive of possible lymphoma (g and h).
The second patient was a 35-year-old woman who had generalized seizures and a 2-month history of paresthesia in the right malar region. The results of the physical examination were normal. Laboratory analyses showed elevated acute phase reactant levels, negative results in serological tests for human immunodeficiency virus, hepatitis B virus and hepatitis C virus, and a negative autoimmunity study. Brain MRI revealed the presence of an intraparenchymal infiltrative lesion affecting left frontoparietal region, with enhanced images following gadolinium administration (Fig. 1.3), which was diagnosed as a brain tumor. Lumbar puncture yielded clear, acellular fluid with slightly elevated protein content and no oligoclonal bands. Treatment was begun with intravenous dexamethasone (12mg/day), which was subsequently tapered. Twenty days later, brain MRI showed a significant reduction in the size of the lesion (Fig. 1.4). 11C-methionine positron emission tomography (PET) of the brain suggested the presence of cerebral lymphoma (Figs. 1.7 and 1.8). The brain mass was biopsied, and the histological study revealed the presence of a focal necrotizing granulomatous infiltrate with lymphocytic vasculitis (Figs. 1.5 and 1.6). Ziehl-Neelsen, periodic acid Schiff (PAS) and silver stains were negative. The results of thoracoabdominal computed tomography, 67gallium scintigraphy and a transbronchial biopsy ruled out sarcoidosis. Three months later, the patient developed oral and genital ulcers. She tested positive for HLA B51. With findings indicative of NBD, treatment was begun with monthly cyclophosphamide pulses (1g/month) plus tapering glucocorticoid doses. After 5 months with this treatment, she had another seizure, and brain MRI performed at that time showed that the lesion had increased in size. As cyclophosphamide was considered to be ineffective, it was replaced by oral azathioprine (2mg/kg bw/day) and the prednisone dose was increased to 30mg/day, after which it was tapered. Four months later, there was no evidence of improvement in the lesion on brain MRI, and the decision was made to start treatment with intravenous infliximab (3mg/kg bw every 8 weeks). After 8 doses of infliximab, there was a significant reduction in the size of the mass, but the patient developed diffuse joint pain and refused to continue the treatment. It was replaced by adalimumab, which she tolerated well. At the time of this writing, 5 years later, she continues to receive subcutaneous adalimumab (40mg/15 days) as single-drug therapy. Her tolerance is well and the brain lesion has not progressed. Over the course of the disease, she has had episodes of oral ulcers that responded to symptomatic treatment.
DiscussionThe clinical presentation and course of NBD complicate the diagnosis of this condition.5 It can present either in the acute form, which responds well to treatment with glucocorticoids and immunosuppressive agents, or in the progressive or chronic form (in which high interleukin 6 [IL-6] concentrations have been reported in cerebrospinal fluid), which is normally resistant to glucocorticoid, cyclophosphamide and azathioprine therapy. These patients have been found to have a better response to methotrexate and/or infliximab.15–17 The presence of lesions compatible with pseudotumors on brain MRI is very rare in NBD, especially as the form of onset of the disease.2,4 The neurological manifestations of BD usually appear from 2 to 4 years after the first clinical sign. However, cases of NBD in which the neurological manifestations preceded other signs of the disease have been reported in the scientific literature.18,19 For this reason, when there is a single brain lesion in the absence of other signs, such as oral and genital ulcers, the diagnosis of BD proves to be complicated.
The differential diagnosis of NBD includes multiple sclerosis, infections, vascular disease and tumors. While both computed tomography and MRI of the brain are sensitive imaging techniques for the diagnosis of lesions, the sensitivity of the latter is greater.19 In brain MRI, the finding is described as a hyperintense signal on T2-weighted images, located predominantly at the diencephalic mesencephalic junction and in the basal ganglia,3,20–22 although the lesion is sometimes found in the periventricular white matter. This makes it difficult to differentiate between NBD and multiple sclerosis. However, multiple locations are reported in the scientific literature (Table 1), and its presentation has even been described as a hypointense mass on T2-weighted images.6 To differentiate it from a tumor in the definitive diagnosis, it is sometimes necessary to perform a brain biopsy, as in the second case reported here.
Clinical Characteristics of the Cases Reported in the Literature.
| Author | Age (years) Sex | History of Behçet's disease (years) | Lesion site | Lumbar puncture | Pathological findings | Outcome | Treatment |
|---|---|---|---|---|---|---|---|
| Litvan14 1987 | 51 M | 30 | Left parieto-occipital region | Increased proteins in cerebrospinal fluid with negative cultures | – | Partial improvement | Glucocorticoids |
| Neudorfer14 1993 | 27 M | No history prior to diagnosis | Right lentiform nucleus | – | – | Improvement | Glucocorticoids |
| Geny26 1993 | – | – | Thalamocapsular region | – | Nonspecific. No evidence of tumor. | Improvement | Glucocorticoids |
| Dupin14 1996 | 20 M | No history prior to diagnosis | Cerebellum | – | – | Improvement | Glucocorticoids, colchicine, azathioprine |
| Visaga27 1996 | 16 F | 2 | From bulbomedullary junction to cerebral peduncle | No | No | Improvement | Glucocorticoids, chlorambucil |
| Yoshimura28 2001 | 41 F | Several | Left thalamolenticular region | Pleocytosis | Nonspecific. No evidence of tumor. | Improvement | Glucocorticoids |
| Ben Taarit31 2002 | 26 F | – | Protuberance and right cerebral peduncle | – | No | Improvement | Glucocorticoids |
| Imoto8 2002 | 50 M | No history prior to diagnosis | Basal ganglia, brainstem and white matter | No | Inflammatory cells and perivascular infiltrate | Reduced size | 3 glucocorticoid boluses |
| Park11 2002 | 52 F | No history prior to diagnosis | Right cerebellar hemisphere extending to 3rd ventricle | Lymphocytes 25/μL, proteins 300mg/dL, glucose normal. No oligoclonal bands. Anti-CMV and herpes simplex negative | Lymphocytic vasculitis | Initial disappearance. Three episodes of recurrence. Death | Glucocorticoids, azathioprine |
| Tuzgen6 2003 | 59 F | No history prior to diagnosis | Right frontotemporal region | No | Gliosis, panvasculitis, thrombosis, extensive infarction and vascular proliferation | Radiological improvement. Persistent left hemiparesis | Surgical excision |
| 45 F | 3 months | Left diencephalic-mesencephalic junction | No | No | Improvement | Glucocorticoids | |
| Bennett7 2004 | 23 M | – | Left temporal lobe, invading peduncle, thalamus, internal capsule, basal ganglia and posterior corona radiata | No | Perivascular inflammatory infiltrate | Clinical and radiological improvement | Glucocorticoids, azathioprine |
| Matsuo12 2005 | 33 M | 7 | Basal ganglia | Pleocytosis 26mm3 (mononuclear), normal glucose and protein levels. No oligoclonal bands | Reactive gliosis with inflammatory infiltrate | Improvement | 3 glucocorticoid boluses |
| Schmolck30 2005 | 39 M | 5 | Left thalamus | No | No | Radiological improvement | Dexamethasone, monthly cyclophosphamide boluses |
| Darmoul10 2006 | 38 M | – | Left thalamus | – | No | Radiological improvement | Glucocorticoids, immunosuppressive agents |
| Appenzeller4 2006 | 43 F | 20 | Right thalamus, lentiform nucleus, subthalamic area and peduncle | No | Gliosis with gemistocytic astrocyte | Disappearance of the mass | Dexamethasone 4mg/day IV, methylprednisolone 1g/3 days IV, oral prednisone 60mg/day, cyclophosphamide |
| Kösters13 2006 | 30 M | No history prior to diagnosis | Frontoparietal region | Normal cell counts, glucose levels and protein levels. No malignant cells | Lymphocytic vasculitis of small vessels | Improvement | Dexamethasone, azathioprine |
| Heo9 2008 | 47 M | 10 | Multiple masses in left pons region and left parietal cortex | No | Perivascular lymphocytic infiltrate, focal necrosis, gliosis, foamy histiocytes | Reduced mass size | High-dose glucocorticoids |
| Varoglu29 2010 | 38 M | – | Mesencephalon, bilateral basal ganglia, posterior limb of internal capsule and centrum semiovale | No | Nontumoral lesions | Reduced lesion size | – |
| Bouomrani32 2010 | 45 M | 17 | Brainstem | – | No | Improvement | Cyclosporine, prednisone, cyclophosphamide |
| Noel14 2012 | 57 F | 11 | Lenticulocapsular | Aseptic meningitis | – | Improvement | Methylprednisolone, cyclophosphamide |
| 47 M | 3 | Peduncles, basal ganglia | – | – | Death | Glucocorticoids, azathioprine, cyclophosphamide | |
| 48 M | 10 | Lenticulocapsular | Aseptic meningitis | Improvement | Methylprednisolone, cyclophosphamide | ||
| 34 M | No history prior to diagnosis | Thalamocapsular | Necrosis with inflammatory infiltrate, and infiltration by neutrophils, lymphocytes and macrophages compatible with vasculitis | Improvement | Methylprednisolone, cyclophosphamide | ||
| 30 M | 3 | Lenticulocapsular | Aseptic meningitis | – | Improvement | Prednisone, cyclophosphamide | |
| Shapiro25 2012 | 30 M | 10 | Cerebral peduncle | Pleocytosis with negative cultures | Good response | Glucocorticoids, infliximab and subsequent tocilizumab with good response | |
| Martínez-Estupiñan24 2014 | 23 M | 4 | Cerebellum | Aseptic meningitis | Reactive changes | Poor response | Glucocorticoids, azathioprine, adalimumab; subsequent tocilizumab, cyclophosphamide, rituximab and plasmapheresis |
–: unknown; F: female; IV: intravenous; M: male.
The histological study of biopsies of brain pseudotumors in NBD reveals a perivascular inflammatory infiltrate, gliosis, necrosis or neuronal loss.7,8,11,23
Treatment with glucocorticoids, as well as the use of other immunosuppressive agents (cyclophosphamide or azathioprine), is accompanied by a rapid response, with a reduction in size or even the disappearance of the lesion.
To date, 27 cases of pseudotumors developing in the course of NBD have been reported in the scientific literature (PubMed: 1987–2014; search terms: neuro-Behçet, pseudotumoral). The Table shows the clinical characteristics of these lesions. The reported cases suggest that pseudotumors develop more often in men (n=18, 66.7%) than in women, with a mean age at presentation of 38 years (range, 16–59 years); the most common site was the thalamus and basal ganglia. All the patients described were initially treated with high-dose oral or intravenous glucocorticoids. Other immunosuppressive agents, such as azathioprine, cyclophosphamide, methotrexate and chlorambucil, were added.
The patient in our first case responded rapidly to treatment with glucocorticoids and azathioprine, whereas the second patient required biological therapy with anti-tumor necrosis factor-α and infliximab at first and, subsequently, adalimumab. This is one of the few cases of the use of biological therapy for the management of these patients reported in the literature.24,25
A pseudotumor in NBD should be considered in the differential diagnosis of brain masses, especially when a good clinical response is achieved after treatment with glucocorticoids. Biopsy may sometimes be necessary to confirm the diagnosis.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Alonso S, Riveros-Frutos A, Martínez-Morillo M, Grau-Ferrer L, Carrato C, Olivé A. Enfermedad de Behçet pseudotumoral. Reumatol Clin. 2016;12:85–90.