The outcome of the SARS-CoV-2 (COVID-19) infection fundamentally affects the lung field, causing ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS). This process is an inflammatory picture, involving an NLRP3 INFLAMOSOME-triggered cytokine storm, the main player in alveolar destruction. IL-1 beta stands out among the cytokines that are triggered in this picture. ANAKINRA is a potent biological drug, capable of blocking this IL 1 beta. We propose its use in controlling ARDS secondary to COVID-19 infection.
El desenlace de la infección por SARS-CoV-2 (COVID-19) afecta fundamentalmente al campo pulmonar, ocasionando un cuadro de SÍNDROME DE DISTRÉS RESPIRATORIO AGUDO (SDRA). Este proceso es un cuadro inflamatorio, protagonizado por una cascada de citocinas bajo el amparo del INFLAMOSOMA NLRP3, responsable principal de la destrucción alveolar. De entre todas las citocinas que se desencadenan en este cuadro destaca la IL beta. ANAKINRA es un potente fármaco biológico, capaz de bloquear esta IL 1 beta. Proponemos su uso, de cara a controlar el SDRA secundario a la infección por COVID-19.
One of the most serious complications in patients infected with COVID-19 is cardiopulmonary failure termed acute respiratory distress syndrome (ARDS).1
Since 1995, several authors have highlighted the central role of IL-1 Beta (IL-1) in the inflammatory cascade of ARDS.2,3 There are 2 publications involving more than 20 patients, showing elevated IL-1β levels in plasma and bronchoalveolar lavage. In addition to these publications, many others describe an increase in countless proinflammatory cytokines (IL-1, IL-6, TNF-alpha, IL-8, etc.) in the alveoli of patients with ARDS.4
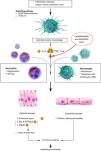
In 2018, Aranda-Valderrama and Kaynar published a model to determine the flow of cytokines and proinflammatory cells in the pulmonary alveoli of patients affected by ARDS.5 They observed how inactive IL-1β (pro-IL-1β) progresses to active IL-1β inside the alveolar macrophages (Fig. 1). This IL-1β generates the activation of the alveolar macrophage, thereby triggering the release of proinflammatory molecules such as IL-6, IL-18, TNF, G-CSF and CCCL2.
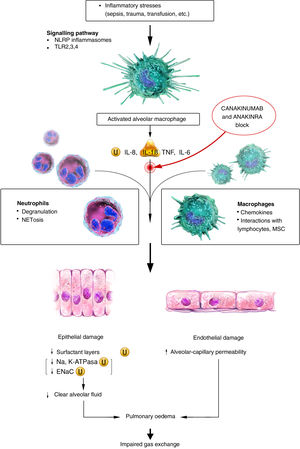
Pathogenesis of acute respiratory distress syndrome (ARDS) is triggered after an initial injury that interacts through alarmin receptors and TLRs that activate nuclear transcription factors, including NF-κB. Subsequently, a potent acute immune response triggered primarily by IL-1ß results in macrophage/neutrophil activation and recruitment. The cell-mediated immune response results in tissue damage, which promotes the development of oedema and epithelial damage.
IL: interleukin; MAPK: Mitogen-activated protein kinase; MMPs: Matrix metalloproteinases; NETs: Neutrophil extracellular traps; PAMPS: Pathogen-associated molecular patterns; ROS: Reactive oxygen species; TLR: Toll-like receptor.
Source: version adapted from the original by Aranda-Valderrama et al. Scientific illustrator: Miguel Soto.
In 2006, He et al. studied the high number of inflammatory cytokines in patients with ARDS secondary to coronavirus (CoV) infection. Autopsies of these patients showed elevated MCP-1, TGF-β1, TNF-α, IL-1β and IL-6.6
This and other publications7 also study the NLPR3 inflammasome and its influence on alveolar macrophage activation in ARDS patients. This is a complex of proteins that interact in a unidirectional manner generating an inflammatory cascade. Innate immunity responds to infection and tissue damage by activating this molecular platform. In human clinical research, four classes of inflammasomes related to inflammatory processes have been described: NLRP1, NLRC4, NLRP3 and AIM-2.8 Of these, NLRP3 is the best studied. Inflammasomes have the common purpose of processing and activating caspase-1, the enzyme responsible for the maturation of pro-IL-1β y and pro-IL-18.9,10
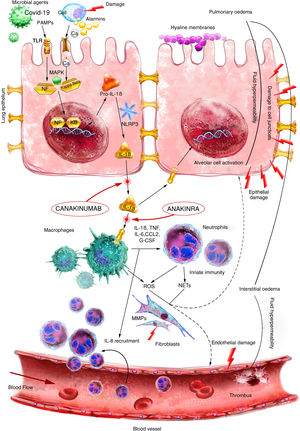
This protein group can detect molecular patterns linked to potential danger, either endogenous (DAMPs) or exogenous (PAMPs) signals that activate various intracellular signalling pathways, mainly through toll-like receptors (TLRs), culminating in the activation of different pro-inflammatory transcription factors such as nuclear factor kappa B (NF-kB) or activator protein 1 (AP-1). These danger signals can also lead to inflammasome activation and maturation of de IL-1β and IL-18 (Fig. 2). The NLRP3 inflammasome can cause epithelial and endothelial damage in the lung parenchyma, leading to severe respiratory disorders such as ARDS and acute lung injury (ALI), possibly resulting in severe pneumonia.11
Pathophysiology of ARDS. The initial inflammatory stimulus activates alveolar macrophages through TLR and NLRP signalling. Activated alveolar macrophages release proinflammatory cytokines and recruit circulating macrophages and neutrophils to injury sites. This influx of persistently activated neutrophils and macrophages causes extensive damage to epithelial and endothelial lung tissue, resulting in an impaired alveolar-capillary barrier.
ENaC: Epithelial sodium channel; Na: Sodium; NETs: Neutrophil Extracellular Traps; TLR: Toll-like receptor; U: Ubiquitination.
Source: version adapted from the original by Seung Hye Han et al. Scientific illustrator: Miguel Soto.
It is likely that COVID-19 infection can trigger a violent immune response and in turn activate the NLRP3 inflammasome in alveolar macrophages, causing a potentially fatal “storm” of cytokines, notably IL-1β.
We currently have a drug called anakinra, which neutralises the biological activity of interleukin-1 alpha and ß by competitively inhibiting their binding to the interleukin-1 type I receptor. Another available molecule is canakinumab, which binds with high affinity specifically to IL-1β and neutralises its biological activity by blocking interaction with IL-1 receptors, thus preventing IL-1β induced gene activation and the production of inflammatory mediators.
These drugs that can block IL-1β activity have shown efficacy in the treatment of autoinflammatory diseases such as neonatal-onset multisystem inflammatory disease (CINCA), Muckle-Wells syndrome (MWS), familial cold autoinflammatory syndrome (FCAS), Still's disease and cryopyrin-associated diseases, a rare genetic disease caused by an autosomal dominant mutation in the NLRP3 gene.12,13
The link between the presence of an NLRP3 inflammasome and the origin of the inflammatory cascade in ARDS has been confirmed in the medical literature.11,12 The initiating pathway of IL-1β makes it a specific and central target for controlling the pro-inflammatory picture generated in the pulmonary alveolus. It is thus a potential therapeutic target in patients who may progress to ARDS after COVID-19 infection. The use of anakinra (anti IL 1β R) or canakinumab (anti IL 1β) is a therapeutic option to control alveolar inflammation secondary to COVID-19 infection.14,15
However, these are different drugs with equally different pharmacokinetics and pharmacodynamics. The peak serum concentration (Cmax) of canakinumab occurs approximately 7 days after a single subcutaneous dose of 150 mg administered to patients with cryopyrin-associated periodic syndrome (adult CAPS). The mean terminal half-life is 26 days and mean peak plasma concentration (Cmax) after a single subcutaneous dose of 150 mg in a typical adult CAPS patient (70 kg) is 15.9 μg/mL and an absolute bioavailability after subcutaneous administration of 66%.15 Anakinra, in contrast, has an absolute bioavailability of 95% for healthy adults after a 70 mg subcutaneous bolus injection. The Cmax of anakinra is generally 3–7 h after subcutaneous administration of clinically relevant doses (1−2 mg/kg/day) and the terminal half-life ranges from 4 h to 6 h. It is therefore a drug with a shorter half-life than canakinumab and thus with great potential for use in a clinical emergency such as ARDS due to COVID-19.14
It is likely that using the immunosuppressive biologic therapies is a way to control ARDS due to COVID-19. However, other scenarios must be considered beyond the individual potential of each molecule where even 2 or more of these lines of treatment can be added. While we need randomised clinical trials to advance the safety and efficacy of anakinra in ARDS due to COVID-19, this option should be considered when current treatments are ineffective or unavailable.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Muñoz-Jiménez A, Rubio-Romero E, Fuente JLM. Propuesta de uso de anakinra en el distrés respiratorio agudo secundario a COVID-19. Reumatol Clin. 2021;17:309–312.