Systemic lupus erythematosus is the diffuse autoimmune connective tissue disease that most frequently involves pulmonary involvement, affecting 20% of 90% of the patients. The percentage varies depending on the defining criteria (symptoms, pulmonary tests or histopathological studies). At least once during the disease course, 50% of those affected have pleural and/or pulmonary manifestations, which are associated with higher morbidity and mortality. Pulmonary involvement has no correlation with lupus activity biomarkers, and it is necessary to rule out infectious processes in the initial approach. Bacterial infection is most frequently the cause of lung involvement in lupus and is one of the most important causes of death. Pulmonary involvement is considered to be primary when it is associated with disease activity, and secondary when other causes participate. Drugs have been reported to be associated with pulmonary damage, including interstitial disease. The incidence of malignant lung diseases is increased in systemic lupus erythematosus. Treatment depends on the type and severity of pulmonary involvement.
El lupus eritematoso sistémico (LES) es la enfermedad autoinmune difusa del tejido conectivo que con mayor frecuencia afecta al pulmón, la cual oscila del 20 al 90%, porcentaje variable en función de los criterios empleados en las cohortes estudiadas (sintomatología hasta histopatología). Más del 50% de los pacientes presentan manifestaciones pleuropulmonares por lo menos una vez durante el curso de su enfermedad y tal afección se asocia a mayor mortalidad. Las anomalías pulmonares no correlacionan con marcadores séricos de actividad lúpica. Es prioritario descartar infección pulmonar en la evaluación inicial, ya que la afección parenquimatosa más frecuente es la infección bacteriana y constituye una de las principales causas de muerte. También se han descrito participación de agentes atípicos, que incluyen los que condicionan enfermedades granulomatosas y otros oportunistas. La afección pleuropulmonar en LES puede estar directamente asociada a LES o ser secundaria. Fármacos pueden ocasionar neumonitis e incluso progresión a enfermedad intersticial. Hay un incremento discreto en el riesgo de neoplasias pulmonares.
Systemic lupus erythematosus (SLE) is the autoimmune disease with the highest prevalence of pulmonary involvement, which ranges from 20% to 90% of the patients, depending on the criteria employed in the cohorts being studied (symptomatology or histopathology).1,2 More than 50% of the patients develop pleuropulmonary manifestations at least once during the course of the disease; likewise, pleuropulmonary involvement has been associated with a higher rate of mortality.3 Symptoms such as pleuritic pain, cough and/or dyspnea are usually the first signs of SLE-related pulmonary involvement, or can be the first manifestation of SLE. Up to 60%4 of the patients have reported dyspnea at least once throughout the disease and abnormal respiratory function tests have been documented in 30%–40%,5–7 as well as anomalies on computed tomographic scans in 55%–70%.7 In the Latin American GLADEL (Grupo Latino Americano de Estudio del Lupus) cohort, at least one pleuropulmonary manifestation was observed in 421 of the 1480 patients included (28.4%).8
Lung anomalies do not correlate with serum markers of lupus activity. It is essential to rule out pulmonary infection in the initial evaluation, as bacterial infection (67%) has been reported to be the most frequent parenchymatous involvement1 and is one of the major causes of death.9 In 2007, Kinder et al.10 reviewed cases of pneumonia in a cohort of SLE patients and identified the etiology to be bacterial in 75%, mycobacterial in 12%, mycotic in 7% and viral in 5%. The participation of atypical agents and/or opportunist pathogens has also been described.11
The risk factors reported for pleuropulmonary involvement in SLE include age at diagnosis of generalized lupus erythematosus (GLE) ≥30 years (odds ratio [OR] 1.42; 95% confidence interval [CI]: 1.10–1.83), presence of lower respiratory tract infections (OR 3.19; 95% CI: 2.05–4.96), nonischemic heart disease (OR 3.17; 95% CI: 2.41–4.18), ischemic heart disease (OR 3.39: 95% CI: 2.08–5.54), systemic (OR 2.00; 95% CI: 1.37–2.91), ophthalmic (OR 1.58; 95% CI: 1.16–2.14) and renal manifestations (OR 1.44; 95% CI: 1.09–1.83),8 hypocomplementemia (relative risk [RR] 3.38; 95% CI: 2.17–5.24) and high titers of anti-double-stranded (ds) DNA antibodies (RR 1.30; 95% CI: 0.78–2.24).12
The conditions that constitute pleuropulmonary involvement in SLE are considered primary when they are directly attributed to SLE or secondary when they are attributable to other causes. Among the latter, infections have a prevalence of nearly 60% and have been responsible for from 30% to 50% of the deaths of patients with SLE.9 Acute respiratory distress syndrome (ARDS) has a variable prevalence of 4%–15% with mortality of nearly 70% and it is mostly secondary to sepsis.
Drugs like methotrexate (MTX) and rituximab can result in pneumonitis and even progression to interstitial lung disease. Likewise, a slight increase in the risk of neoplasms in general, pulmonary in particular, has been reported in SLE patients.2
The disorders that constitute pleuropulmonary involvement in SLE are grouped according to the structures affected:
- •
Parenchymal involvement2:
- ∘
Lupus pneumonitis: prevalence from 1% to 12%.
- ∘
Chronic interstitial lung disease: prevalence from 3% to 13%.
- ∘
Diffuse alveolar hemorrhage (DAH): prevalence from 2% to 6%, high mortality.
- ∘
- •
Pleural involvement: 50%–70%, in the form of pleuritis and/or effusion.2
- •
Vascular involvement:
- ∘
Pulmonary hypertension: prevalence of 0.5%–4.2%.13
- ∘
Embolism/pulmonary thromboembolism (PTE): deep venous thrombosis with/without PTE in 9%, related to activity. The presence of antiphospholipid antibodies (in up to 30% of the patients with SLE) increases the risk of thromboembolic events by 35%–40%.14
- ∘
Acute reversible hypoxemia: a rare condition that is characterized by unexplained hypoxemia with no evidence of parenchymal involvement. Its pathophysiology is controversial, but leukocyte aggregation and complement activation in the pulmonary vasculature have been proposed. It has a good response to steroids within 72h.3
- ∘
- •
Shrinking lung syndrome: prevalence of 0.6%–0.9%, characterized by unexplained dyspnea, elevated diaphragm and reduced lung volumes without interstitial involvement. The etiology is controversial but phrenic neuropathy, inflammatory myopathy and, more recently, pleural disease have been suggested. It responds well to steroids.14
- •
Airways: both lower and upper, with a prevalence that ranges from 0.3% to 30%; its course is predominantly subclinical.3
Below we review the epidemiological, clinical, diagnostic and treatment aspects concerning the conditions that the authors considered to be of particular interest due to their high frequency or because of the severity they represent.
Pleural InvolvementPrevalence. Pleuritis constitutes the most widespread thoracic manifestation in SLE. Pleuritic pain is present in 45%–60% of the patients, pleural effusion in up to 50% and, in autopsies, it has been reported to be encountered in up to 93%.2 The experience documented in the GLADEL cohort is similar; pleural involvement was the most common pleuropulmonary manifestation (24%),8 and it was observed that ischemic and nonischemic heart disease (OR 2.99; 95% CI: 2.33–3.82 and OR 1.99; 95% CI: 1.28–2.09, respectively) constitute risk factors. Other factors include the presence lupus nephritis, hypocomplementemia (C3 and C4) and high levels of anti-dsDNA antibodies.12 A recent study that included 119 patients with SLE and pleural involvement demonstrated that, even in regions in which tuberculosis is endemic, the main etiology of pleural effusion in these patients continues to be the underlying disease (52%).15
Clinical aspects. The main symptom is pleuritic pain, usually accompanied by fever, cough and dyspnea. On occasion, pleural effusion is asymptomatic and can only be detected by radiography. The effusions are usually small and bilateral, although they can also be unilateral. They tend to be evanescent and recurrent.16
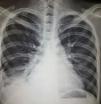
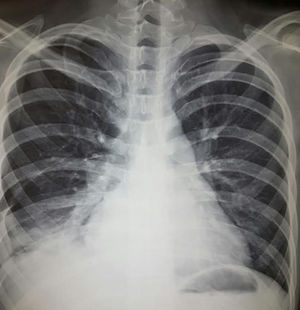
Diagnostic aspects. The differential diagnosis should include musculoskeletal pain, pulmonary embolism, infection, heart failure, uremia and neoplasm. Analysis of the pleural fluid is the main diagnostic tool. This typically is an exudate with a slight elevation of leukocytes, predomination of mononuclear cells (there are also polymorphonuclear cells) and normal or slightly low glucose levels. The differential diagnosis should include rheumatoid arthritis (RA), characterized by a higher level of leukocytes and lactate dehydrogenase, as well as a low glucose level.2 The role of the detection of antinuclear antibodies (ANA) in pleural fluid for the diagnosis of lupus pleuritis is controversial. Two recent studies17,18 have reported ANA at titers >1:160 with sensitivity of 85%–90% and specificity of 80% for the diagnosis of lupus pleuritis in patients with lupus; however, high titers can also be encountered in other conditions. Fig. 1 shows evidence of pleural effusion in a patient with lupus who reported clinical data associated with pleural involvement.
A 22-year-old woman with a 3-year history of generalized lupus erythematosus characterized by skin and musculoskeletal manifestations. She presented with a 1-month history characterized by pleuritic-like pain and dyspnea. Plain chest radiography revealed radiopaque images that obliterated the costophrenic angles, compatible with pleural effusion. It was contended with and was subsequently attributed to the activity of her underlying disease.
Pleural biopsy. Rarely performed, usually only when the diagnosis is uncertain. The findings are nonspecific: lymphocytic and plasma cell infiltration, fibrosis and fibrinous pleuritis. Immunofluorescence shows a nuclear pattern with anti-IgG, anti-IgM and anti-C3.16
Complications. Usually benign course, although a case was reported in which the patient developed fibrothorax with severe restriction; the response to pleurectomy was favorable.19
Treatment. Most patients respond to nonsteroidal anti-inflammatory drugs (NSAID) or oral steroids (prednisone at 10–30mg/day). Azathioprine has been employed as a steroid-sparing agent and cyclophosphamide only when there is concomitant systemic involvement. Pleurodesis with tetracycline or talc has been employed in large recurrent effusions.2
Acute PneumonitisPrevalence. A number of series describe a variable prevalence of between 2% and 9%.20–25 Five corroborated cases have been reported over the past 8 years. In the GLADEL cohort, it was reported in 2.3% of the patients and was associated with nonischemic heart disease (OR 2.48; 95% CI: 1.20–5.13).8
Clinical aspects. Acute and nonspecific onset: cough with or without hemoptysis, dyspnea and fever. In severe cases, there can be hypoxemia and even acute respiratory failure. It can be the initial manifestation of SLE in up to half of the patients who develop it.26 The characteristic radiographic image consists of uni/bilateral alveolar infiltrates, usually with predominance at the lung base.27 Wan et al. recently reported a series of 5 cases in which lupus pneumonitis constituted the initial manifestation; 100% had pulmonary images on radiographs, chest computed tomography (performed in 2 patients) showed ground glass opacities and bilateral patchy consolidation. All had additional manifestation of GLE that enabled the diagnosis: mucocutaneous in 100%, hematological in 80%, serositis in 40% and 60% were positive for anti-dsDNA antobodies.28
Initial management and diagnosis. The main condition to be included in the differential diagnosis is infection; it is necessary to initiate empirical antibody coverage after the collection of cultures to deescalate or discontinue it if infection is ruled out.27
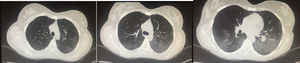
Association with high titers of anti-dsDNA antibodies has been described. The utility of bronchoalveolar lavage (BAL) lies in that it rules out infection; characteristically, there is an increase in cellularity at the expense of activated polymorphonuclear cells.3 The extraction of a biopsy specimen can be limited to cases of uncertain diagnosis for the exclusion of alternative etiologies. The findings are nonspecific: damage to the alveolar wall and necrosis, infiltration by inflammatory cells, edema, hemorrhage and hyaline membranes.16 A number of experts question the existence of this syndrome unless one or more of the following findings are demonstrated: interstitial fibrosis, vasculitis, hematoxylin bodies, interstitial pneumonitis, alveolitis or pleuritis.27 It is useful to remember that, in terms of the diagnosis, the establishment of lupus pneumonitis is nearly always simultaneous with a flare of the disease, in general with multiple organ involvement (e.g., renal involvement and serositis) and, in most cases, this occurs in the presence of anti-SSA antibodies (82%). Thus, the combination of pneumonitis with multiorgan involvement in a patient who is positive for anti-SSA supports the diagnosis.29Fig. 2 shows an example of a computed tomography from a patient with lupus pneumonitis.
A 24-year-old woman with a 4-year history of generalized lupus erythematosus characterized predominantly by musculoskeletal and hematological manifestations (autoimmune hemolytic anemia). She came to the emergency department with a 10-day history consisting of dyspnea and dry cough and later on with hemoptysis on 6 occasions. At admission, she had tachycardia and hypoxemia. Computed tomography revealed predominantly bibasal ground glass. After an infectious process was ruled out, the possibility of lupus pneumonitis was considered. She received pulses of methylprednisolone and cyclophosphamide and improvement was achieved.
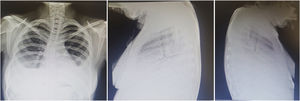
Prognosis. Historic series report a mortality of up to 50%; the most recent series reported a mortality rate of 40%.28 Although it is an uncommon complication, lupus pneumonitis during pregnancy and the postpartum has been associated with especially adverse outcomes.29–31 The predominance of lymphocytes in BAL is associated with a more favorable course, whereas the predominance of eosinophils or neutrophils is associated with a higher rate of mortality. In up to 50% of the survivors, interstitial infiltrates and abnormal respiratory function persist, with risk of progression to chronic interstitial pneumonitis.3,14Fig. 3 shows the findings in pneumonitis and pulmonary hypertension.
A 22-year-old man with a twin brother with systemic lupus erythematosus. Both showed evidence of Schnitzler syndrome (intermittent episodes of fever, urticaria, abdominal pain, angioedema, arthritis). He had a 2-month history of progressive dyspnea even on minimum exertion, polyarthritis, hypertension, mechanical edema and, on the preceding days, cough and increased dyspnea. Exploration revealed Chávez’ pulmonary complex and right basal condensation syndrome; radiograph showing evidence of pulmonary hypertension, 4 arches, right basal pneumonitis and homolateral effusion. He was diagnosed with lupus pneumonitis, which was managed with prednisone at 0.5mg/kg body weight/day and cyclophosphamide, with a satisfactory response.
Treatment. There are no controlled studies; the current approach is based on case reports. Broad-spectrum antibiotic coverage is indispensable until infection is ruled out. The cornerstone of treatment is prednisone at a dose of 1 to 1.5mg/kg body weight (bw)/day.27 If there is no response in 72h, the recommendation is the administration of intravenous pulses of glucocorticoids (methylprednisolone at 1g/day for 3 days); it is necessary to consider adding an immunosuppressive agent like cyclophosphamide. Patients with significant tachypnea or hypoxemia and/or those in whom alveolar hemorrhage is suspected should be admitted to the intensive care unit, and it is essential to employ methylprednisolone pulses as the initial treatment.2 There are reports of cases of different degrees of success with azathioprine, intravenous gammaglobulin, plasmapheresis and rituximab.2,32,33 In the retrospective review by Wan et al., all the patients received high doses of a glucocorticoid, 80% intravenous cyclophosphamide and 60% intravenous immunoglobulin.28
It has been observed that influenza infections can activate and exacerbate GLE in general and lupus pneumonitis, as well. Although the humoral response to an influence vaccine is weaker than in the general population, vaccination in patients with GLE with low-to-moderate activity is moderately effective and safe. The available information does not warrant our making recommendations in reference to GLE with high activity.34
Chronic Interstitial Lung DiseasePrevalence. Reported in from 3% to 8% of the population, it has been observed that it increases with disease duration.35 Several cohorts of SLE patients have found a reduction in carbon monoxide diffusion capacity in 27%–56%, a restrictive pattern in 8%–80%6,7,36 and interstitial involvement in high-resolution computed tomography (HRCT) in 30%–40% of those included; however, the majority are asymptomatic (50%–90%).5,7,36,37 This suggests that there is a subclinical course in most of those affected. The known risk factors include disease duration of >10 years, Raynaud's phenomenon, anti-U1 ribonucleoprotein antibodies, sclerodactyly, capillaroscopic changes and older age.38
Clinical aspects. It usually predominates in men over 50 years of age with late-onset or long-standing (>10 years) SLE.26 The course is usually insidious, but it can develop after one or more episodes of acute pneumonitis. It generally consists of dyspnea on exertion, occasional pleuritic pain, dry cough and bibasal rales.2 In the early stages, radiographic images may be normal or show irregular linear opacities. Later studies show diffuse or bibasal infiltrates, pleural disease, honeycombing and reduced lung volumes. High-resolution computed tomography defines the presence and the pattern of the disease.39 The severity of the lung involvement does not correlate with the serological markers.16 However, the association of anti-Ro/SSA has been reported in up to 80% of the patients.40 This suggests a possible contribution of secondary Sjögren's syndrome. Lymphoid interstitial pneumonia (LIP) is associated with Sjögren's syndrome in up to 53%; there are reports of LIP in SLE without and with secondary Sjögren's syndrome.41,42
Diagnosis. The following are included among the available diagnostic tools:
- •
High-resolution computed tomography: corroborates the presence of interstitial disease and makes it possible to classify it in accordance with the patterns observed, which are highly similar to those encountered in systemic sclerosis35,39:
- ∘
Nonspecific interstitial pneumonia (NSIP): patches of ground glass and reticular pattern.
- ∘
Usual interstitial pneumonia (UIP): reticular opacities with predominantly subpleural and basal honeycombing, with or without traction bronchiectasis.
- ∘
Lymphoid interstitial pneumonia (LIP): less common. Diffuse ground glass opacities, reticular pattern and perivascular cysts.
- •
Respiratory function tests: document the extension and progression. Restrictive pattern, reduced carbon monoxide diffusion capacity and exercise-induced desaturation are characteristic.26,35
- •
Bronchoalveolar lavage: its main utility lies in that it excludes other etiologies. The relationship to the prognosis is not well-established. The analysis of the BAL fluid in asymptomatic patients suggests subclinical alveolitis.26 A study conducted by Chhajed et al. in patients with SLE and RA demonstrated a predominance of macrophages, followed by lymphocytes and neutrophils, as well as a relationship between lymphocytic predominance in individuals with pulmonary symptoms and predominance of neutrophils and lymphocytes in those with radiological evidence of interstitial lung disease.43
- •
Biopsy: recommended if the diagnosis is uncertain despite noninvasive studies.26,35
- ∘
NSIP: the most common type. We stress the chronic lymphocytic infiltrate (lymphocytes/plasma cells), absence of fibrosis.
- ∘
UIP: patches of fibrosis with remodeling of lung architecture, moderate chronic interstitial inflammation.
- ∘
LIP: diffuse interstitial inflammatory infiltrate marked by lymphocytes, plasma cells and histiocytes.
- ∘
Less common associations with amyloidosis and cryptogenic organizing pneumonia have also been reported.
Treatment. There are no controlled clinical trials. There are small studies that initially showed a favorable response and an increase in the carbon monoxide diffusion capacity with prednisone at a dose of 60mg/kg bw. The rest of the therapeutic approaches have been extrapolated from those utilized in systemic sclerosis due to the similarity of the interstitial involvement.3,44 In cases in which the severity is low-to-moderate, prednisone has been employed as monotherapy, but it has more frequently been associated with another immunosuppressive agent. In severe or progressive disease, the treatment is prednisone at 1–2mg/kg bw and cyclophosphamide, with a subsequent change to azathioprine or mycophenolate mofetil.14 The real-world experience with cyclophosphamide is limited; there are case reports involving MTX and, recently, with rituximab, both with favorable responses.45–48Prognosis. The disease course is variable but the changes occur slowly; it is rarely progressive and the trend is toward stabilization over time.26 A historical series reported insignificant changes in the main parameters of respiratory function tests.49 Patients with evidence of scleroderma or with overlap syndromes usually have a higher prevalence of restriction.26 In accordance with the histological pattern, in a series of biopsies including patients with RA, Sjögren's syndrome, polymyositis and SLE, the most common pattern was NSIP (41%), with a mortality rate of 24%; the most frequently associated pattern was the fibrotic form.50
Diffuse Alveolar Hemorrhage (DAH)Prevalence. It is variable, from 0.6% to 5.7%; however, survival continues to be unacceptably low in most reports.51–57 In 2011, researchers from our group documented a prevalence and survival of 9% and 49.7%, respectively.58
Pathophysiology. It has not been completely clarified. Evidence suggests that the disease activity plays a crucial role since most of the series report high Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores (>12), concomitant high prevalence of nephritis (66%–100%), arthritis (15%–75%) and neuropsychiatric manifestations (20%–60%), as well as polymorphonuclear leukocyte infiltration and, occasionally, immune complex deposition in capillaries (capillaritis) and alveolar wall, although the majority of the patients had “bland” hemorrhage (blood in alveoli without inflammation or destruction of the wall).60 This, in accordance with the series of autopsies of SLE patients reported in 1981 and 2009, suggests that processes like aspiration, infection, heart failure and renal failure may largely contribute to the pathogenesis of DAH.1,61 At the present time, we recognize 3 histopathological patterns that can frequently overlap: pulmonary capillaritis (neutrophilic infiltration of alveolar septa, loss of capillary structural integrity and passage of erythrocytes into alveolar spaces and interstitium; in some cases, the participation of antiphospholipid antibodies has been implicated in this process), bland pulmonary hemorrhage (accumulation of erythrocytes and fibrin in alveolar spaces without inflammation or destruction of alveolar structures) and diffuse alveolar damage (edema of alveolar septa and formation of hyaline membranes).62
Risk factors. They have been poorly described, but a strong association has been established with disease activity; particularly with class III and class IV lupus nephritis (90%),60,63 high titers of anti-dsDNA antibodies, low complement levels, SLEDAI score >1057 and neuropsychiatric manifestations.56
Clinical aspects. The majority of the cases described occur in women, the duration of SLE prior to DAH is variable (6 months–14 years) and it can be the initial event in 10%–30%.54,64 It consists of dyspnea, decreasing hemoglobin, changing radiographic infiltrates and hemoptysis may be present (it is absent in up to 30%–50% of the patients).65 The radiographic features that suggest the presence of DAH include the sudden development of infiltrates, poor response to antibiotics and the rapid radiographic resolution after management with corticosteroids.54 Bilateral diffuse alveolar infiltrates have been found in most patients; on occasion, alveolo-interstitial infiltrates are observed, that may be unilateral in 20% or patchy in 40%. High-resolution computed tomography is the most sensitive technique for the proper assessment of the pattern and distribution of DAH, as well as the associated pulmonary changes.40,65
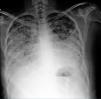
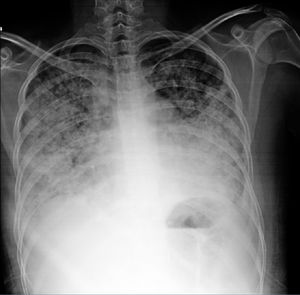
Diagnosis. It can be corroborated by the clinical findings, the unexplained decrease in hemoglobin, the characteristic radiographic findings and the favorable response to treatment. Fig. 4 is a radiograph showing alveolar images characteristic of alveolar hemorrhage. Certain tools that may contribute to the diagnosis include:
- •
Carbon monoxide diffusion capacity: some series report an increase of 30% over the baseline or a rise of >130% over the predicted value. Difficult to perform or not viable in most patients because of their critical condition.51
- •
BAL: confirms or rules out infection and the presence of hemosiderophages. Useful in patients with nonspecific manifestations.54,59
- •
Biopsy: rarely performed due to the morbidity and mortality rates in critical patients. Capillaritis and depositions of immune complexes and neutrophils in alveolar walls have been described in 14%. The predominant finding is bland hemorrhage (72%).66,67
A 20-year-old woman with a 4-year history of systemic lupus erythematosus. She had necrotizing vasculitis in lower limbs, psychosis, erythema, photosensitivity, thrombocytopenia, lymphopenia, severe anemia, was positive for Venereal Disease Research Laboratory and had an antinuclear antibody titer of 1:640, homogeneous and peripheral. In recent months, she had developed progressive dyspnea even on minimum exertion; over the last few days, she had increased cough, dyspnea and hemoptysis. Her hemoglobin level was 3g, and she had hypoxemia, nephrotic proteinuria and telescoped sediment. The chest radiograph shows alveolar images compatible with diffuse alveolar hemorrhage. The patient received 1g methylprednisolone for 3 days and cyclophosphamide and the response was satisfactory.
The differential diagnosis should consider: pulmonary embolism, heart failure, lupus pneumonitis and infection, the latter two are among the principal conditions.2
Infection. Recent studies have shown that concomitant pulmonary infection is present in up to 60% of the patients with DAH53; likewise this factor is associated with a higher rate of mortality. The microorganisms commonly reported include Pseudomonas spp, Aspergillus, Staphylococcus aureus and cytomegalovirus.59 In the cohort studied by our group,68 which included 50 patients, we reported a mortality of 42% and infection in 38%. The factors associated with a higher risk of infection were: mechanical ventilation, hypocomplementemia and high C-reactive protein levels. In contrast, in patients who had had no previous treatment for SLE, we observed a lower frequency of infectious processes.
In a previous article by our group, we stressed the importance of a systematic search for infection in all patients with DAH, given the possibility of opportunists and even the need for thoracoscopy to collect a biopsy specimen, taking into account the risk-benefit in each case. Likewise, it is necessary to institute empirical broad-spectrum antibiotic coverage as part of the initial management.54,56
Prognosis and mortality factors. Episodes of DAH can recur and survivors may continue to have persistently abnormal respiratory function tests. The mortality reported 30 years ago was as high as 90%; over the past 15 years it ranges between 40% and 60%.59 Among the factors associated with a higher rate of mortality, we stress the presence of concomitant pulmonary infection, renal failure, the need for mechanical ventilation, thrombocytopenia and a high Acute Physiology and Chronic Health Evaluation (APACHE II) score; the last 4 factors are those most frequently associated.51,54–56,69
Treatment. As in the other conditions described above, there are no controlled clinical trials and the current approach to treatment derives from case reports and case series. The following include those that are most widely employed59:
- •
Intravenous pulses of methylprednisolone of 1g/day for 3 or more days until clinical improvement is observed, which is associated with a longer survival.70
- •
Cyclophosphamide: initially associated with a higher rate of mortality (employed in patients who were more severely ill); however recent series have demonstrated no effect or that it served to protect against mortality.54,55,63
- •
Plasmapheresis: usually together with pulses of methylprednisolone and cyclophosphamide in cases of poor response; its utility alone has not been specified, but a reduction in the mortality of 20% has been documented.59
- •
Immunoglobulin: employed in a case series, with no impact on mortality.54
- •
Rituximab: employing immunoregulatory mechanisms other than B-cell depletion,71 it has a favorable and relatively rapid response that is observed in case series and isolated case reports, particularly with a reduction in recurrence.72–78
- •
There are small series and case reports with variable degrees of success with therapies that include transplantation of umbilical cord-derived mesenchymal cells, extracorporeal membrane oxygenation, factor VIIa and mycophenolate mofetil.79–86
Pulmonary involvement in SLE is highly prevalent (although a significant proportion of the patients may be asymptomatic). It can affect virtually any component of the respiratory system and may constitute the initial manifestation of SLE. In contrast to what we observe in other organs (skin, kidney, nervous system), pleuropulmonary involvement has a poor relationship or none at all with serological markers. The manifestations are similar, being nonspecific and their severity is variable.
The differential diagnosis is extensive and the main suspect is an infectious process, which constitutes the main cause of death in SLE. Thus, it is essential to institute broad-spectrum antibiotic coverage in the initial approach.
The current lack of controlled clinical trials limits the available therapeutic armamentarium for potentially fatal conditions such as acute pneumonitis and/or DAH; it is not likely that there be future studies of this nature due to the relative rarity of these disorders and their severity. The available therapeutic agents are, on occasion, quite unsatisfactory and their utilization in the severe pulmonary manifestations associated with SLE is derived mainly from case series and from experience obtained in other rheumatic diseases (like interstitial involvement in systemic sclerosis).
The role that biological agents could have in these severe manifestation still continues to be under evaluation; we hope to obtain more information in this respect that derives from the use of these agents in case series.
Conflicts of InterestThe authors declare they have no conflicts of interest concerning the present publication.
Carlos Abud-Mendoza has been a lecturer for BMS, Pfizer, UCB and Roche and has occasionally participated in workshops as an advisor.
Please cite this article as: Aguilera-Pickens G, Abud-Mendoza C. Manifestaciones pulmonares en lupus eritematoso sistémico: afección pleural, neumonitis aguda, enfermedad intersticial crónica y hemorragia alveolar difusa. Reumatol Clin. 2018;14:294–300.