To describe the optimal therapeutic strategy for use of methotrexate in RA patients over the initial dose, route of administration, dose increase and decrease, patient monitoring, and use of folic/folinic acid.
Materials and methodsEleven clinical experts proposed some questions to be solved. A systematic literature search was conducted. The contents were selected in a work session and subsequently validated via email to establish the level of agreement.
ResultsThe initial dose of methotrexate should not be <10mg/week, preferably orally, but the parenteral route is considered as an alternative due to compliance, non-effectiveness of treatment or gastrointestinal side effects, polypharmacy, obesity (if required doses are >20mg/week), patient preference, very active disease or to avoid administration errors. Changing to a parenteral administration is proposed when the oral route is not effective enough, gastrointestinal toxicity appears, there is non-compliance or due to cost-effectiveness reasons before using more expensive drugs. On the contrary, due to patient preferences, intolerance to injections, dose reduction <7.5mg/week, non-effectiveness of the route, poor compliance or gastrointestinal side effects. There should be a rapid dose escalation if inadequate responses occur up to 15–20 or even 25mg/week in about 8 weeks, with increments of 2.5–5mg. The reduction will be carried out according to the dose the patient had, with decreases of 2.5–5mg every 3–6 months. Patient monitoring should be performed every 1–1.5 months until stability is reached and then for every 1–3 months.
ConclusionsThis document pretends to solve some common clinical questions and facilitate decision-making in RA patients treated with methotrexate.
Describir la estrategia terapéutica óptima de uso de metotrexato en AR sobre dosis inicial, vía de administración, incremento y disminución de dosis, seguimiento del paciente y uso de ácido fólico/folínico.
Material y métodoOnce expertos plantearon los interrogantes clínicos a resolver. Se realizó una búsqueda bibliográfica sistemática. Los contenidos fueron seleccionados en una sesión de trabajo y el nivel de acuerdo se estableció posteriormente en una ronda de consenso vía correo.
ResultadosLa dosis de inicio de metotrexato no debe ser < 10 mg/semana, preferentemente por vía oral, considerando la vía parenteral como alternativa según el cumplimento, ineficacia o efectos secundarios gastrointestinales, polimedicación, obesidad (si requiere dosis > 20 mg/semana), preferencias del paciente, enfermedad muy activa o para evitar errores de medicación. Se cambiará a la vía parenteral cuando haya ineficacia, toxicidad gastrointestinal, incumplimiento o por coste-efectividad antes de pasar a fármacos más caros; y a la inversa, según preferencias del paciente, intolerancia a inyectables, reducción de dosis < 7,5 mg/semana, ineficacia, bajo cumplimiento o efectos adversos gastrointestinales. Se realizará escalada rápida de dosis si la respuesta es inadecuada hasta los 15-20 o, incluso, 25 mg/semana en unas 8 semanas, con incrementos de 2,5-5 mg. La reducción se realizará según la dosis a la que estuviera el paciente, con disminuciones de 2,5-5 mg cada 3-6 meses. El seguimiento del paciente deberá realizarse cada 1-1,5 meses hasta la estabilidad y luego cada 1-3 meses.
ConclusionesEste documento pretende resolver algunos interrogantes clínicos habituales y facilitar la toma de decisiones en la AR tratada con metotrexato.
The correct use of disease-modifying antirheumatic drugs (DMARDs) has improved the prognosis of RA based on its early use and setting a specific therapeutic goal, aiming to reach remission or the lowest possible degrees of inflammatory activity.1–3 In this sense, EULAR recommends that DMARD treatment should begin as soon as the diagnosis of RA is made.4
Methotrexate (MTX) has been used for over 20 years in the treatment of rheumatoid arthritis. The features that make the MTX as the DMARD of first choice are related to its low price, its favorable safety profile, its slowing of radiographic progression, clinical experience with high response rates, therapeutic continuity, availability and versatility of doses and routes of administration. Thus, after the diagnosis of the disease, it is indicated as the first-line treatment for early and in clearly defined RA, as recommended by different societies4–6, and it is also suitable as an anchor drug for combination therapies.7
However, there is a large variability in the clinical practice in our country with regard to the starting dose, the rate and pattern of dose escalation, selection of routes of administration and dosing with concomitant use of folic acid or folinic acid in patients with RA. This is because the clinical practice may not be clearly supported by appropriate clinical trials.
The objective of this study is to establish recommendations for decision-making in the treatment of adult RA with MTX, based on scientific evidence.
Materials and MethodsFor the preparation of the consensus document a group of experts, who drafted the recommendations (ER), was made up by 11 rheumatologists from hospitals of the Spanish National Health System. The ER worked according to the methodological basis given by the Quality Plan of the Ministry of Health, in its reference document “Development of clinical practice guidelines in the National Health System”.8
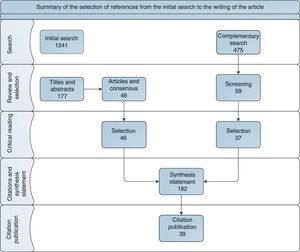
The ER work in the context of the use of MTX in the treatment of adult RA was performed with a participatory and structured methodology, in which ER members identified 3 major blocks: (a) indication criteria; (b) initial dose and route of administration and changes along the treatment; (c) criteria for dose modification and withdrawal of MTX. A detailed index was established to solve clinical questions, to which they later applied the PICO methodology, in order to facilitate and manage the process of literature search and the subsequent development of specific recommendations developed in response to each issue raised. The systematic review of the literature was used as the basic source of the original query, clinical trials, cohort studies, protocols, review articles and clinical guidelines. Filter criteria and the search limits were established by the ER panel. The objective of all of this was to make feasible an updated and critical reading of the key aspects of the scientific evidence available. A search for articles published since 1995 was conducted, both in English and Spanish, in databases such as PubMed, Cochrane Bookseller, Trip data base and abstracts of the congresses of the Spanish Society of Rheumatology (2005–2012), The European League Against Rheumatism (2012) and the American College of Rheumatology (2010–2011) and in the latest update available for MTX data sheets for both oral and parenteral formulations (Fig. 1).
The initial search result yielded 1241 articles. Relevant articles were selected in 2 phases. The first prioritized titles and abstracts (177), and the second prioritized consensus statements, specific systematic reviews and most recent articles (46). The ER panel considered necessary, in very specific cases, a complementary search in certain areas of therapeutic care, which were not sufficiently defined in the initial search strategy (Table 1). Because of the predominance of review articles and clinical guidelines, references to the summary report of the literature were expanded to a total of 182 citations.
Results of the Expanded Search for Specific Topics.
| Block | Matrix | Screening | Independent selection |
|---|---|---|---|
| 1. Definitions and Interpretation. The efficacy and toxicity of DMARDs (hydroxychloroquine, and leflunomide) with MTX | 84 | 20 | 16 |
| 2. Use of aspirin at antiplatelet doses in combination with MTX | 16 | 7 | |
| 3–5. Use of MTX when desiring pregnancy, during pregnancy and lactation | 166 | 9 | 4 |
| 6. Use of MTX in intercurrent infection, HCV, HBV, HIV, and tuberculosis | 42 | 14 | 1 |
| 7. Use of MTX in minor and major surgery | 123 | 5 | 1 |
| 8. MTX use in vaccination | 13 | ||
| 9. Downscaling: in association with a biological, what should be reduced first? | 17 | 1 | 1 |
| 10. MTX dose fractioning | 14 | 1 | 0 |
| Total articles | 475 | 59 | 37 |
The categorization of levels of evidence (LE) and degrees of recommendation (DR) was performed from system criteria Scottish Intercollegiate Guidelines Network (SIGN)9 themes that used diagnostic criteria of the “Center-based medicine evidence of Oxford “(CMBE). For recommendations that already had NE and/or in other previous documents DR originals are kept, referencing it in each case.
The executive summary of the evidence was assessed in a participatory classroom session, in which 37 recommendations and 14 were proposed evidence. This document was sent to the ER members for their individual assessment and to vote on their agreement, to regard these recommendations as appropriate responses to the clinical questions posed. Finally, 12 recommendations reached an agreement (A) greater than 70% were established as validated formal recommendations.
ResultsFrom the literature search and the articles referred, prioritized by the ER members specifically, this document regarding answers to clinical questions raised was developed, with the objective to reduce the variability in the use of MTX in the treatment of adult RA. The 12 recommendations that reached greater agreement among members of the ER panel are here presented. Of these, 10 have their scientific basis in 28 of the references cited, while 2 correspond to recommendations based from experience and expert opinion of the ER panel.
What Dose Methotrexate Should be Initiated in the Treatment of Rheumatoid Arthritis?It is known that the effectiveness of MTX is dose dependent. Results of some studies indicate that an initial dose of 7.5mg/week was often ineffective and, in many cases required an increase in dose after 6 weeks.10,11 In addition, a starting dose of 12.5–20mg/week was more effective than 5–7.5mg/week, with no differences in safety.12 Therefore, some experts recommend a minimum initial dose of at least 10mg/week, although it is more complicated to establish agreement on what to recommend as an optimal initial dose. However, a systematic review13 defends the optimal initial dose of 10–15mg/week orally, taking into account the specific characteristics of each patient. Based on these evidences, a dose not lower than 10mg/week is recommended; but also lower doses may be considered depending on the circumstances of the patient (comorbidities, low weight, age, renal function, liver).
Recommendation 1: For RA, a starting dose of MTX not less than 10mg/week should be determined, based on the severity of the disease and prognostic factors associated with the patient14 (LE: 1b/2 [Shekelle],15 DR: C [Shekelle],15 A: 82%).
What Route of Administration Should Methotrexate be Initially Delivered Through?The selection of the initial route of administration may be agreed upon between the doctor and the patient. Generally, the patient usually prefers the oral route. Different studies show that the relative bioavailability of oral MTX compared to the intramuscular MTX is good at low doses but decreases at higher doses (Table 1).16–18 Considering that the relationship between oral bioavailability and parenteral dosage is 1 for total doses of 7.5mg, it becomes 0.85 (range 0.77–0.93) when the total dosage reaches 10–15mg and drops to 0.64 (range 0.21–0.94) at doses of 15–20mg.Therefore, a dosage under 20mg/week, ease of handling and low cost of oral MTX position it as the preferred route of administration in RA14; however, after 15mg/week there is no evidence that the oral route is better than parenteral.
However, there are situations in which to consider the parenteral route as the route of choice from the start: polypharmacy patients who are overweight or obese (as administered doses are higher), low compliance, per patient preference in order to reduce certain gastrointestinal adverse effects or to avoid medication errors. In addition, experimental data seem to indicate that the subcutaneous (sc) onset is especially useful in patients with active, longstanding disease (DAS 28≥4).19
Recommendation 2: In patients with RA it is recommended to start treatment with MTX preferably through an oral route. However, consideration should be given to sc or intramuscular routes in patients with poor compliance, insufficient effectiveness or gastrointestinal 14 side effects (LE: 4 [SIGN],9 DR: D [SIGN],9 A: 91%).
When Should the Clinician Change the Route of Administration?Change From Oral to SubcutaneousSome studies have suggested that patients with poor compliance, inadequate effectiveness and/or gastrointestinal side effects (when MTX was administered orally) should be considered when switching to injection20–23 because better results and effectiveness may be achieved. Changing to sc from oral in patients with a lack of response or toxicity has proven useful, with responses reaching 30% higher in the ACR20 criteria.19 In addition, this change in patients with inadequate response could be cost-effective because it can prevent or delay therapy with biological agents.23–25
Recommendation 3: In patients with RA treated orally with MTX, available evidence justifies the change to sc route of administration when a lack of therapeutic response is expressed against the activity of the disease, or gastrointestinal toxicity or therapeutic failure are present, since the sc is associated with better treatment response25 (LE: 4 [SIGN],9 DR: D [SIGN],9 A: 100%).
Recommendation 4: Its better cost-effectiveness in studies suggest the suitability of changing from oral administration to parenteral MTX in patients with an inadequate response, as the evidence shows that this prevents or delays subsequent therapy with biological agents23,25,26 (LE: 4 [SIGN],9 DR: D [SIGN],9 A: 82%).
Change From Subcutaneous Oral RouteThere are reasons for the change from SC to oral: sc administration intolerance, patient preferences, dose reduction to less than 7.5mg or therapeutic failure.
Furthermore, it has been observed that if after switching to oral from sc, one desires to return to the sc route, the response rate may be lower compared to the first batch of parenteral MTX.19,21
Recommendation 5: Some clinical-care criteria can justify changing the route of administration from sc to oral MTX in RA patients: patient preferences, intolerance to the sc route of administration, dose reduction to levels <7.5mg/week, or low compliance14 (LE: 4, [SIGN],9 DR: D/√ [SIGN],9 A: 82%).
How Should the Increase in Dose of Methotrexate be Made? Pattern and TimingSome studies show that initial doses of 25mg/week orally or rapid initial dose escalation therapeutic strategies of 5mg/month to 25–30mg/week are associated with greater efficiency; although with increased toxicity compared to the initial patterns of 5–15mg/week or slow escalation strategies of 5mg/3 months.12,27,28 These results support that achieving high doses (25–30mg/week) in a short period of time is effective, but may produce more adverse events.13 It should also be noted that other studies with more aggressive strategies versus conventional increase patterns showed no statistically significant differences in therapeutic response.29,30
Moreover, in the dose range of 2.5–30mg/week of MTX, it is easy to reach the dose titration with small increments of 2.5mg offering even new formulations for parenteral administration. These are useful for problems of tolerance due to rapid dose escalation for lack of efficacy with the oral formulation.31,32
After evaluating this evidence, the panel of experts validated 2 recommendations:
Recommendation 6: Once the initial dose of MTX has been established, if an adequate response in patients with RA is not achieved, proceed to a rapid increase of the dose up to 15–20 or even 25mg/week in about 8 weeks. Before making a dose increase, one should establish a clinical-therapeutic 4-week observation period, with the preceding dose to determine whether or not it is effective. In case of insufficient clinical response, increments of 2.5–5mg every 2–6 weeks are recommended, depending on the clinical severity, up to a maximum of 25mg weekly24 (LE 5 [Oxford],33 DR: D [Oxford],33 A: 100%).
If no response is seen after 8 weeks with a high dose of MTX, treatment should be discontinued, as indicated by the MTX insert.
How Should the Clinician Reduce the Dose of Methotrexate? Pattern, Timing and Route of AdministrationDue to the paucity of evidence related to MTX dose reduction, the experts agreed to develop their own recommendations. The aim of downscaling is reaching the minimum effective dose to maintain a complete remission (DAS 28<2.6).
Recommendation 7: Downscaling is recommended when complete remission is maintained for a period of time, depending on the dose:
- •
Dose ≥25mg/week: dose reduction will begin when there is sustained remission for at least 6 months.
- •
Doses <25mg/week: dose reduction will begin when there is sustained remission for at least 6–12 months (LE: 4 [SIGN] 9, DR: D/√[SIGN] 9, A: 91%).
Recommendation 8: In patients with RA, as a general guideline for the downscaling of MTX, recommended reductions of 2.5–5mg every 3–6 months should be carried out (LE: 4 [SIGN],9 DR: D/√[SIGN],9 A: 91%).
As mentioned above, previous studies19 show the consequences that could result from changes in route of administration. Therefore, it is agreed that:
Recommendation 9: We recommend keeping track of the patient at the time of decision making (LE: 4 [SIGN],9 DR: D/√[SIGN],9 A: 100%).
However, doses below 15mg are candidates for oral treatment. Therefore, it is agreed:
Recommendation 10: Arriving at doses of <15mg, it is considered appropriate to provide a change from sc to oral, except in the case of prior intolerance to oral or when there is suspected improvement in the efficiency related to the route of administration (LE: 4 [SIGN],9 DR: D/√[SIGN],9 A: 91%).
However, on downscaling during a change of parenteral to oral administration, it may be necessary to increase the oral dose to 2.5–5mg/week with respect to the parenteral dosage to maintain the effectiveness of the treatment.
What Are the Recommendations for Evaluation and Monitoring for Patients With Rheumatoid Arthritis Receiving Methotrexate?Intensive monitoring of inflammatory activity and safety of treatment of RA in the early stages of its evolution is related to better results in remission and control of inflammatory disease activity.13,34,35 From the ACR36 guidelines regarding the appropriate periodicity in the monitoring of these patients, Visser et al.13 established parameters to review treatment as well as its periodicity, although for the latter there is less evidence.
Recommendation 11: To initiate the process of increasing MTX dose, analytical control of ALT with/without AST, creatinine and CBC should be performed every month or month and a half until a stable dose is reached. From the clinical and therapeutic standpoint, monitoring is recommended every 1–3 months, with possible side effects and changes in risk factors monitored at each visit13 (LE: 4 [Oxford],33 DR: C [Oxford],33 A: 91%).
What Should Folic Acid Supplementation be in Patients With Rheumatoid Arthritis Treated With Methotrexate?MTX reduces inflammation by a mechanism related to folic acid metabolism. In some people treated with MTX there may be a deficiency in folic acid, so supplementation with folic acid or folinic may improve tolerability and safety of MTX (oral ulcers, gastrointestinal upset, diarrhea, hematological disorders, elevated transaminases). Folic or folinic acid is administered as one tablet of 5mg a week separating the drug at least one day with regard to the administration of MTX.37,38
Routine supplementation with folic/folinic acid generally does not affect the effectiveness of MTX, although the data of one study suggest that the use of these supplements could lead to a slight increase of the dose of MTX to maintain efficacy.39
Recommendation 12: In patients with RA treated with MTX, administration of 5mg of folic or folinic acid/week is recommended, separating the intake of it from MTX by 24h14 (LE: 1a/1b [Shekelle],15 DR: A [Shekelle],15 A: 100%).
DiscussionThis consensus has been undertaken with the intent to facilitate decision making in the clinical practice of physicians involved in the management of patients with RA treated with MTX, the cornerstone of treatment of RA. Optimization is a process used to modify a system in order to increase its efficiency with the use of available resources. This has clearly been the goal of our consensus, trying to raise the profile of clinical use of this drug.
Based on previous clinical questions, we have found evidence available on the initial dose of MTX used,10,19–26 the route of administration (and possible exchangeability)14,19, therapeutic scaling,12,14,24,27,28,31,32,35 and optimal patient monitoring with MTX.13,36 Based on these reports, the panel of experts has made the appropriate recommendations, emphasizing issues such as who should start at full doses and quickly scale back to quickly gain control of the disease. We also emphasize the usefulness of the proper evaluation of the route of administration, at all times adapting to the characteristics of the individual and the clinical status of the disease.
In conclusion, this paper gives some recommendations on the management of MTX in RA patients, and especially in the process of dose decreasing, which may facilitate clinical decision making and standardize the different ways to proceed.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that this research did not perform experiments on humans or animals.
Data confidentialityThe authors declare that they have followed the protocols of their workplace on the publication of data from patients and all patients included in the study have received sufficient information and gave written informed consent to participate in the study.
Right to privacy and informed consentThe authors state that no patient data appears in this article.
FundingThe study has been developed through an unrestricted grant offered by Gebro Pharma SA.
Conflicts of InterestDr. Jesus Tornero Molina has received payments for research projects and conferences from Abbvie, Gebro, Pfizer and Roche.
Dr. Jordi Carbonell states having an advisory contract with Gebro.
The remaining authors declare that they have no conflict of interest related to the article.
We thank Manel Alepuz and Veronica Albert, of Gestió Organització Comunicació, SA for their support in conducting the methodology used to prepare the study.
Please cite this article as: Tornero Molina J, Ballina García FJ, Calvo Alén J, Caracuel Ruiz MÁ, Carbonell Abelló J, López Meseguer A, et al. Recomendaciones para el uso del metotrexato en artritis reumatoide: incremento y reducción de dosis y vías de administración. Reumatol Clin. 2015;11:3–8.