To update the recommendations for the management of patients with Spondyloarthritis (SpA) in the Mexican population, and identify which variables could influence patient management.
Material and methodsA group of 14 experts in SpA translated, analyzed and modified the recommendations of the Mexican College of Rheumatology (CMR) and the International Society for the Assessment of Spondyloarthritis (ASAS)/European League Against Rheumatism (EULAR) 2016 group through a systematic review of the literature by two external reviewers during the period from 2015 to 2018 using the grade of recommendation, Oxford levels of evidence, percentage of concordance (Delphi).
ResultsCompared to previous recommendations, there were no significant changes from the year 2015. However, we modified the five fundamental principles and reduced the number of recommendations to ten by incorporating the first item in the text and combining five recommendations into two and adding a further recommendation. We confirmed the tendency to use glucocorticoids for patients with inflammatory activity and scarce access to biologicals. We identified the sociodemographic and clinical characteristics of patients with SpA and their influence on the application of the recommendations.
ConclusionsThe ten recommendations of the CMR and the analysis of the characteristics of the Mexican patients with SpA focussed on step therapy, including pharmacological and non-pharmacological therapies, in a spectrum from easily accessible to high-tech substances available to a small percentage of the population.
Actualizar las recomendaciones para el manejo de pacientes con Espondiloartritis (EspA) en la población mexicana e identificar las variables que podrían influir el manejo del paciente.
Material y métodosUn grupo de 14 expertos en EspA tradujo, analizó y modificó las recomendaciones del Colegio Mexicano de Reumatología (CMR) y el grupo Sociedad Internacional de Evaluación de las Espondiloartritis (ASAS)/Liga Europea contra el Reumatismo (EULAR) del 2016 a través de la revisión sistemática de la literatura por dos revisores externos en el período de 2015 a 2018 utilizando los niveles de GRADE y Oxford y el porcentaje de concordancia (Delphi).
ResultadosEn comparación con las recomendaciones anteriores, no hubo cambios significativos desde el año 2015. Sin embargo, modificamos los cinco principios fundamentales y reducimos el número de recomendaciones a 10 por la incorporación de la primera en el texto, la combinación de cinco recomendaciones en dos y la adición de una nueva. Confirmamos la tendencia del uso de glucocorticoides para pacientes con actividad inflamatoria y escaso acceso a productos biológicos. Se identificaron las características sociodemográficas y clínicas de los pacientes con EspA y su posible influencia en la aplicación de las recomendaciones.
ConclusionesLas diez recomendaciones del CMR y el análisis de las características de los pacientes mexicanos con EspA se centran en el tratamiento escalonado con medios farmacológicos y no-farmacológicos, fácilmente accesibles o por el contrario sustancias de alta tecnología para un pequeño porcentaje de la población.
Recommendations or guidelines for the management or treatment of rheumatic diseases have become fundamental documents for identifying diseases and assessing clinical and therapeutic manifestations based on evidence. The benefit of this type of document is reflected in the timely control of the disease, the health of the patient, better use of resources and reduced treatment costs.1,2
In 2005, we published a review of the literature on the efficacy and safety of biological drugs in patients with ankylosing spondylitis (AS).3 One year later, the Mexican College of Rheumatology (CMR) Guidelines and Recommendations for the Use of Biologic Agents in Rheumatic Patients included rheumatoid arthritis, psoriatic arthritis (PsA), juvenile idiopathic arthritis, generalized lupus erythematosus and in particular AS.4oIn that study we found that 61% of Mexican experts agreed with the proposal made by the International Society for the Assessment of Spondyloarthritis (ASAS) and the European League Against Rheumatism (EULAR) for the initial administration of biological disease-modifying antirheumatic drugs).5 We then surveyed 253 rheumatologists certified by the Mexican Council of Rheumatology A.C. and found that over 75% of them agreed with the 10 recommendations of ASAS/EULAR.6
This document updates the recommendations for the treatment and management of radiographic (r-axSpA) and non-radiographic (nr-axSpA) axial spondyloarthritis (axSpA) and peripheral spondyloarthritis (pSpA), without including PsA, based on the recommendations developed and published by the ASAS/EULAR group in 2016.7 This paper also fulfils our role as promoters of these recommendations, as this document deals with educational and academic aspects for doctors and the need for information and knowledge about the patient to make decisions together with their families and physicians. Endorsed by the CMR, these recommendations constitute the official position of our group in the most important proposal for their implementation in the relevant health systems.
Material and methodsGroup of expertsA group was formed of 14 rheumatologists with expertise and publications in any of the following areas: axSpA, recommendations and therapeutic guidelines, clinical practice in the country’s various states and health systems. The executive committee (EC) comprised the principal investigators, the project coordinator and two spokespeople. Its functions were the planning and executing of the project, preparing the work agendas, and editing the recommendations. The systematic review of the literature (SLR) was carried out by two independent investigators.
The EC selected the expert team, themes and sub-themes related to the definition, concept and classification of SpA, demographic and clinical similarities or discrepancies compared to other populations, including their overall impact. It also developed the clinical and research questions using the ASAS/EULAR8 model, but not limited to it, to carry out the initial SLR.
Systematic literature reviewThe EC and independent investigators converted the clinical questions into research questions covering the following theme: inflammatory activity, structural deterioration, improvement, partial remission, inactive disease, moderate activity, high activity, very high activity, significant clinical improvement, very significant clinical improvement, quality of life and health status. In addition, they chose to retrieve information on the safety and efficacy of pharmacological, non-pharmacological and surgical treatments from studies after the SLR conducted for the ASAS/EULAR update in 2016.7
The literature search in the databases was conducted between January 2015 and March 2018, considering that most of the information prior to January 2015 had been obtained, analysed and published by ASAS/EULAR.7–9 Meta-analyses, systematic reviews, controlled and randomised clinical trials, controlled clinical trials and extension clinical trials identified in PubMed, Ovid-Medline, Cochrane Library, Scopus, Science Direct and CINAHL were chosen.
In accordance with the PICO (Patient or problem, Intervention, Comparison, Outcomes) structure, article eligibility was based on: 1) patients >18 years with diagnosis of SpA; 2) pharmacological therapeutic intervention with conventional synthetic or biological synthetic disease-modifying antirheumatic drugs (csDMARDs or bDMARDs, respectively), non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids and others, non-pharmacological (exercise and information programmes) and surgical; 3) comparison of interventions with different regimen or dose, placebo or none; 4) outcomes, including Ankylosing Spondylitis Disease Activity Score (ASDAS), Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI), improvement criteria ASAS 20, ASAS 40, ASAS 5/6, ASAS-partial remission (PR), pain, C-reactive protein (CRP), radiographic indexes such as the Bath Ankylosing Spondylitis Radiology Index (BASRI), modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS), Radiographic Ankylosing Spondylitis Spinal Score (RASS), and MRI indices from the ASAS Task Force, Berlin and Leeds, and the Spondyloarthritis Research Consortium of Canada (SPARCC).10–18
The quality of evidence of the articles was assessed according to the Grading of Recommendations, Development and Evaluations (GRADE) document, based on risk of bias, consistency, direct results, heterogeneity, accuracy and publication bias, and the Oxford Centre for Evidence-Based Medicine’s (OCEBM) levels of evidence.19,20
Working meetingsIn the first meeting, the experts presented the main characteristics and definitions in the management of SpAs to the plenary of the committee, discussed the circumstances and therapeutic options that exist in Mexico, and gave indications for the SLR based on different research questions.
In the second, the results of the SLR were reviewed and the 13 recommendations from the ASAS/EULAR update were followed in a structured way, discussing the current status of the evidence.7 The study coordinator presented each theme, sub-theme and recommendation to the plenary; she then gave the floor to each of the experts so that in less than 5 minutes they could give their reasons for voting for or against the proposal. The vote followed the Delphi technique in a positive or negative way according to the following levels (>80% in the first round, <75% in the second and <60% in the third). A priority order of recommendations was followed with adaptation to knowledge of the national context, experience of the experts and updated evidence.
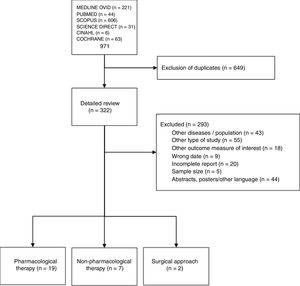
ResultsSystematic literature reviewWe obtained 322 articles to review in depth (Fig. 1). Of these, 28 articles met the criteria to be included in the final review: 19 on pharmacological treatment (Appendix B supplementary material, Annex 1), 7 on non-pharmacological therapy and 2 on surgical approach (Appendix B supplementary material, Annex 2).
Fundamental principles and recommendationsThe ASAS/EULAR format includes 5 fundamental principles for the management of patients with SpA and 13 recommendations developed from them. Although this format is present in all the sets of recommendations developed by EULAR, our group preferred not to separate and to include the fundamental principles as part of the text of the recommendations because, although they deal with generic principles, we did not consider that there was much difference with respect to the individual recommendations.
The meaning of the updated questions and answers were discussed. The information concerning recommendations 1, 2, 3, 4, and 5 exceeded the capacity of the SLR and was not sufficient to change their meaning. On the other hand, we did not find substantial updated information relating to recommendations 6, 7, 8, 9, 10, 11, 12 and 13. Thus, the SLR that included the articles from the last 2 years did not result in important changes regarding the ASAS/EULAR 2016 recommendations.7 However, the Mexican group of experts made the recommendation, modification, change or substitution of some of the ASAS/EULAR recommendations clear in Table 1, which shows the 10 Mexican recommendations and the percentage of concordance.
Mexican recommendations for the management of spondyloarthritis.
| Recommendation | Level of evidence | Grade of recommendation | Agreement (%) | |
|---|---|---|---|---|
| 1 | Individualise the treatment of AxSpA according to signs and symptoms present at consultation (axial, peripheral and extra-articular manifestations), characteristics of the patient, comorbidities, and psychosocial factors | 5 | D | 80 |
| 2 | Monitor the effect of treatment as reported by the patient, clinical findings, laboratory tests and imaging studies obtained with appropriate instruments. The frequency of monitoring will be individual, depending on the symptoms, their severity and treatment | 5 | D | 100 |
| 3 | Guide treatment to a previously determined therapeutic target | 5 | D | 100 |
| 4 | Promote patient learning about the disease; encourage participation in activities involving constant exercise and discouraging smokinga | 2, 5, 1a | B, D, A | 100 |
| 5 | Avoid the prescription of csDMARDs in patients who only have axial symptoms. Consider the use of sulfasalazine in patients with peripheral arthritis | 4 | C | 100 |
| 6 | Prescribe NSAIDs - first line of treatment, maximum recommended dose, taking risks and benefits into account - for the reduction of pain and joint stiffness. In patients who improve, prescribe NSAID treatment according to the recurrence of symptoms. Prescribe paracetamol and opioid-like drugs in case of residual pain when other treatments are contraindicated or poorly tolerated | 1a, 5 | A, D | 80 |
| 7 | Consider the application of injectable glucocorticoids to sites of musculoskeletal inflammation, specifically joints, sacs and synovial sheaths. Recommend the use of oral or intramuscular glucocorticoids in patients with persistent, highly disabling inflammatory activity. In both cases, the prescription of glucocorticoids will only be recommended when treatment with NSAIDs, opioid-like analgesics, rest, physical therapy or rehabilitation is contraindicated or poorly tolerated by the patient and especially when the patient does not have access to bDMARDsb | 2, 5 | B, D | 100 |
| 8 | Consider the use of bDMARDs in patients with persistent activity despite having received conventional treatments. So far, treatment is usually started indiscriminately with TNFi and IL-17Ai as the first line. In patients who have not improved or who have relapsed, the use of different TNFi or IL-17Ai should be considered. Consider reducing bDMARDs if the disease activity is in a stage of sustained remission. Consider prescribing “biocomparable medicine” as soon as relevant information about their efficacy and safety is available | 1a, 1b | A | 80 |
| 9 | Consider hip replacement and corrective vertebral osteotomy in patients with persistent pain and disability, severe radiographic abnormalities, regardless of age, in centres with staff with experience | 4 | C | 100 |
| 10 | Consider the socio-economic situation, educational level and opinion of the patient and his/her family members in the process of deciding how to manage the patient's problems | 4 | C | 100 |
Levels of evidence. Evidence from 1a: Meta-analysis of randomised controlled trials; 1b: At least one randomised controlled trial; 2a: At least one non-randomised controlled trial; 2b: At least one quasi-experimental study; 3: Descriptive studies; 4: Report, opinions and/or clinical experiences of authorities (experts) in the theme in question; 5: Any of the above, useful for reporting degree of inconclusive recommendation regardless of level of evidence.
Grade of recommendation. A: Level 1 studies 1; B: Level 2 or 3 studies or extrapolations from level 1 studies; C: Level 4 studies or extrapolations from level 2 or 3 studies; D: Inconsistent or inconclusive evidence from studies of any level.
Results from different supervised exercise programmes, home, and others, have been reported in recent years. The results are still inconsistent in terms of design, however, they all present favourable outcomes (Appendix B Supplementary material, Annex 2).
In Mexico, access to bDMARDs is limited, the experts in this study agreed that they are an alternative that should be shared. Source: Based on the recommendations issued by ASAS/EULAR, the 2006 consensus document of Mexican rheumatologists and the systematic review of the literature of the last 2 years and meeting of experts held in 2018.
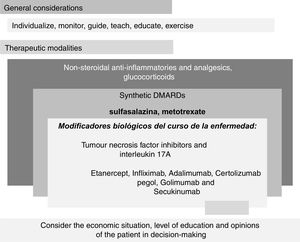
In addition, through the analysis of the 10 recommendations, the information was stratified in the model known as palimpsest (Fig. 2).
“Palimpsest" style diagram of the Mexican recommendations for the management of spondyloarthritis resulting from the text of Table 1. The diagram is divided into two main parts, "General" and "Therapeutic modalities". The first relates to the fundamental principles; the second, to the therapeutic modalities.
Unlike the traditional flow chart in which the substitution of one therapeutic modality for another is a constant and therefore the changes are absolute and stepped - for example, the patient who did not improve with anti-inflammatory drugs switches to synthetic DMARDs; and if he/she does not improve with these either, they are replaced by biological DMARDs, going from one step to another - our diagram is based on aggregation and overlapping recalling the palimpsest style. With this model, therapeutic recommendations and changes are presented in a format close to clinical practice. In general, such a change involves adding and perhaps later substituting therapeutic modalities. For example, we do not substitute prednisone for sulfasalazine, nor this for etanercept before the loss of efficacy of the first and the certainty of the efficacy of the second have been demonstrated, but we add a new modality with greater efficacy to another already used in which the expected efficacy was not obtained. According to expert opinion, the proportion of patients who are treated with the drugs listed in recommendations 5 to 8 is much higher than the proportion who are treated with surgery. Also, the proportion receiving sulfasalazine is higher than the proportion receiving synthetic DMARDs. Although it seems contradictory, synthetic DMARDs do not reduce or stop the progressive worsening of the disease. With respect to biologics, the anti-inflammatory effect may be so great that the speed and deterioration in the structure of the joints and entheses is less. Recommendation 10 is of great importance since it refers to the access of the population with the disease to the most effective, safe, and remission-inducing treatments.
The themes relating to the profile of Mexican patients with SpA cover definition, concept and classification, demographic and clinical similarities, or discrepancies. In part, the purpose of this section was to investigate the characteristics of our population and structural and logistical aspects to determine whether there was compatibility with the therapeutic recommendations of the ASAS/EULAR group.
I. Definition, concept, and classification. The change in the definition and concept of the ASAS group was considered. AxSpA is the defining term for the entity characterized by acute and chronic inflammation in the synovial membrane and bone proliferation in the entheses of the sacroiliac, intervertebral, interapophyseal joints and peripheral sites.7,21,22 The predominant manifestations determine AxSpA21,23,24 or the peripheral form (p-SpA),25 alterations in the sacroiliac joints, in the radiographic, specifically AS,16,21,26 and non-radiographic (nr-SpA) varieties.21,23 The entities included in the SpA group continue independently, specifically PsA, reactive arthritis and arthritis associated with inflammatory bowel diseases and undifferentiated SpA. Changes in classification, specifically PsA, have not been accepted by all experts.27,28 In this paper, we adopt the classification of the ASAS group,23,25 we retain the nomenclature, definition and classification of SpAs made by the European Spondyloarthropathy Study Group (ESSG)29 and the definition of AS according to the modified New York criteria.26
II. Relevant socio-demographic and clinical manifestations: comparison with other populations. In our country, AxSpA occurs more frequently in males with the histocompatibility antigen HLA-B27 and aged around 30 years at the onset of symptoms.30–33 Among females, the clinical spectrum is broader and includes peripheral site involvement, overlap with fibromyalgia and less response to TNFi (tumour necrosis factor inhibitor). In comparison with European and Latin American populations, it has been observed that in the latter two, there is a higher prevalence of axial symptoms and lower in the peripheral sites, and greater age at the onset of symptoms.30–35 However, the group of experts considered that there are generally no important differences between the SpAs identified in the rest of the world.
III. Epidemiological impact. The prevalence of SpA in Mexico is .6% (95%CI: .4, .9); that of AxSpA is .3% (imaging studies approach) and .09% (HLA-B27 approach).36 The prevalence of AS is .1% (95%CI: .02, .2). AxSpAs are among the top 5 causes of care in specialist clinics.37 The prevalence of HLA-B27 in the general population and in healthy controls in association studies is 1%-4%.38,39
IV. Functional capacity, quality of life and health status. In a significant percentage, the effect of SpA on functional capacity, quality of life and health status is a burden affecting the patient and his/her environment. Studies, both inside and outside healthcare institutions, have reported that the cost of SpA is high when measured through out-of-pocket expenses and impoverishment, direct and indirect costs, and finally temporary and permanent disability. The health status of patients with AxSpA is lower compared to the general population (accepted for publication). More than half the patients with SpA in Mexico consider their health status to be poor.40,41
V. Survival and comorbidity. No precise information is available on survival. The most frequent concomitant diseases are osteoporosis, peptic acid disease and cardiovascular involvement.42,43 Although not exactly known, given the high prevalence of obesity and diabetes, in addition to the underdiagnosis of high blood pressure in Mexico, it is possible that there is a major prevalence of metabolic syndrome in Mexicans with SpA.44
VI. Access to high efficacy and high cost treatment. It is a fact that there is no therapeutic equity in our country. About 50% of the Mexican population has access to health systems with full coverage such as the Mexican Social Security Institute, the Institute of Security and Social Services for State Workers, Petróleos Mexicanos, the Ministry of National Defence, prepaid systems to banks and other organisations and systems with state and municipal coverage. However, the other half of the population does not have a medical service to cover the cost of these types of diseases. For these people, there is the popular insurance and the Ministry of Health which do not have the capacity to pay for chronic disease such as SpA and many others. The cost of this type of treatment is borne by the patient and their family; in the case of rheumatoid arthritis and possibly SpA, the higher the percentage of out-of-pocket expenditure in families, the greater their impoverishment.39,40,45
DiscussionWith the support of the CMR, a group of experts on the clinical aspects, assessment and treatment of the SpAs met and developed the corresponding recommendations based on the analysis, correction, editing and cross-cultural adaptation of those published by the ASAS/EULAR group in 2016.7 In 2006, more than 75% of 253 rheumatologists certified by the Mexican Council of Rheumatology agreed with the 10 recommendations of ASAS/EULAR.6 The current Mexican recommendations are in line with previous findings and the current recommendations.
Major work has been done on disseminating and adapting the recommendations of the ASAS/EULAR group since their first edition.46–54 Our version did not elaborate on the 5 fundamental principles, a "European" section that appears in all the therapeutic recommendations in which EULAR participates, as this proved to be superfluous for us. The wording of the Mexican recommendations is simple and specific, making them accessible to most physician and non-physician stakeholders involved in administrative and decision-making tasks.
Finding 6 of the themes that are of most influence in the management of patients in Mexico with SpA enabled us to assess the disease and its repercussions and to conceptualise SpA in a high proportion of young adults with signs and symptoms in joints and peripheral entheses, which has a great impact on health status, quality of life and expenditure due to disease.40 Unfortunately, there was agreement that limited access to current therapies in Mexico is an important determinant of the health status of the patient.
Despite advances in treatment, since the previous edition of the ASAS/EULAR criteria, we did not find any significant changes. Most of the differences related to the addition of two recommendations and the inclusion of the treat-to-target scheme (T2T). In this regard, it is important to point out that T2T is controversial at the moment as the treatment target is not defined in the SpAs because it could actually refer to two asynchronous phenomena: inflammation as an initial phenomenon and bone proliferation as a late phenomenon. While the use of bDMARDs can reduce inflammation by blocking pro-inflammatory cytokines, the latter has not yet been achieved.55
The SLR of the last 2 years that fuelled the present consensus included new elements: 1) the platform with data on our country’s patient profile; 2) the use of glucocorticoids; 3) the efficacy of the first IL-17A blocker, secukinumab, and 4) the possibility of using “biocomparable medicines” as part of treatment. In addition, the SLR incorporated certain clarifications in some recommendations (Table 1). These include the lack of efficacy of csDMARDs, which excludes the possibility of treatment with sulfasalazine, methotrexate and leflunomide. However, about 40% of rheumatologists worldwide seem to be convinced of its efficacy in one way or another.56–58
On the other hand, and unlike ASAS/EULAR, Mexican rheumatologists consider that oral and parenteral administration of glucocorticoids has an important place in the management of the SpAs. However, it should be noted that our group discourages the use of infiltrations or injections of glucocorticoids into entheses, tendon sheaths or tendons. This is not the case inside the joints or synovial sacs where, in the opinion of the experts, they are effective in the case of inflammation. Furthermore, systemic use (oral or parenteral) of glucocorticoids is indicated in severe cases that do not respond to usual treatment, such as ocular, intestinal, and renal manifestations. The dose and duration of treatment depend on the site involved.
Although, in general, the guidelines for the treatment of AS of the American College of Rheumatology, the National Association of AS and the Spondyloarthritis Research and Treatment Network reach the same conclusions as the recommendations of the ASAS/EULAR group, we were struck by the fact that the systemic use of any form of glucocorticoids is prohibited in patients with AS.59 The experience of the rheumatologist in Mexico indicates that oral, parenteral and local glucocorticoid routes result in decreased intensity of inflammation and associated symptoms, especially in patients with arthritis and peripheral enthesitis, and even more so in cases of inflammatory conditions of the spine and sacroiliac joints.6 It is important to highlight that the controversy about the use of glucocorticoids is not supported by evidence but by individual experience, especially in patients without access to bDMARDs. The recommendations of the Spanish Society of Rheumatology for the use of TNFi and other biologic agents essentially follow this trend.60
The ASAS/EULAR recommendations devote three sections to the use of bDMARDs to which the Mexican counterpart devotes only one, which includes a brief note about biosimilars. The criteria for starting treatment with bDMARDs remain the same: 1) diagnosis of SpA (by a rheumatologist), and 2) increase in serum concentration of CRP and/or sacroiliitis on MRI (bone oedema in sequences with fat suppression) and/or X-rays (grade 2 bilateral or 3 or 4 unilateral). In addition, to have used two NSAIDs for 2 weeks each in all patients; in those with peripheral manifestations, to have tried local injection and sulfasalazine for a reasonable time without improvement. Finally, a high degree of activity should be confirmed with ASDAS (greater than 2.1) and BASDAI (greater than 4).5
TNF blockers and the IL-17A inhibitor are in the first line of bDMARD treatment. In terms of controlling inflammatory activity and associated symptoms, they have been shown to be effective in the short and long term. However, some reports with little evidence put the loss of efficacy of TNFi over the years down to unclear mechanisms, among which is the neutralization of the biologic’s molecule by antibodies.61,62 Even so, switching to another drug with the same target or with the IL-17A inhibitor is justified.
The SLR of publications in the last 2 years confirmed the efficacy and safety of the TNFi and IL-17 inhibitors compared with placebo. In most patients, the beneficial effect of TNFi is maintained in the long term.63–66 On X-ray, worsening appears to be delayed; on MRI, improvement is significant. Interpretation of these data was difficult, but while the effect of the biologic on inflammation remains remarkable, the effect on deterioration from a radiographic point of view is still uncertain (Appendix B Supplementary material, Annex 1).
Sustained remission, from 6 to 12 months, measured through the criteria of partial remission of ASAS or the ASDAS index could be considered sufficient reason to reduce the dose or to extend the interval between applications as a first step in the interruption or definitive discontinuation of the biologic.62–67 However, relapse has not yet been defined, much less the criteria for re-treatment. Regarding the use of “biocomparable medicine”, there is still scant information, the comparison of infliximab and its biosimilar are essentially reported, and no significant differences between the groups have been found.68
Finally, two articles cover the correction of serious deformities in the spine using different techniques. The results reported have been good, but onset of severe complications is relatively frequent (Appendix B Supplementary material, Annex 1).
ConclusionsThe 10 recommendations for the management of patients with SpA cover the analysis, correction, editing and cross-cultural adaptation of the recommendations recently published by ASAS/EULAR and those arising from the discussion of the Mexican experts. We believe that the original recommendations and those incorporated by the CMR reflect the profile of patients with SpA in our country.
We hope that this edition not only responds to the scientific treatment of the problem, but that the Mexican authorities have the demographic, socio-economic and clinical elements to implement it in the Mexican health system.
FundingThe company Novartis provided funding to the "Executive Committee for the elaboration of recommendations for the management of spondyloarthropathies" for transport, accommodation, search, and bibliographic analysis expenses. We further declare that none of the members of the committee for the elaboration of recommendations for the management of spondyloarthritis or members of the board of directors of the Mexican College of Rheumatology received any remuneration or payment.
Conflict of interestsThe authors have no conflict of interests to declare.
The committee for the development of the Mexican Recommendations for the Management of Spondyloarthritis expresses its gratitude to the Mexican College of Rheumatology AC, to Dr Wayra Paz and Dr Joaquín Herrera from the company Elsevier for the analysis and literature review, and to the company Novartis for their unrestricted support of the work of our committee.
Please cite this article as: Reyes-Cordero G, Enríquez-Sosa F, Gomez-Ruiz C, Gonzalez-Diaz V, Castillo-Ortiz JD, Duran-Barragán S, et al. Recomendaciones del Colegio Mexicano de Reumatología para el manejo de las espondiloartritis. Reumatol Clin. 2021;17:37–45.
The position of the Mexican College of Rheumatology with respect to terminology is to use "innovative biotechnological medicine" and "biocomparable medicine" instead of "biological" and "similar". http://go.medicaleconomics.com/acton/attachment/12262/f-000a/1/-/-/-/-/BIOCOMPARABLES2015-FINAL-JAT6.pdf
- Home
- All contents
- Publish your article
- About the journal
- Metrics
- Léalo en español
- Download PDF
- Bibliography
- Additional material