Hydroxychloroquine is used in the long-term therapy of systemic lupus erythematosus (SLE). Although considered to be a safe treatment, side effects have been documented. An uncommon side effect is thrombocytopenia. In order to establish the diagnosis of thrombocytopenia secondary to Hydroxychloroquine, non-pharmacological causes must be ruled out and it is necessary to determine a recurrence after re-exposure to the drug. We present one case of severe thrombocytopenia occurring in a patient with SLE undergoing treatment with Hydroxychloroquine.
La hidroxicloroquina es un fármaco antimalárico, empleado como terapia a largo plazo en el lupus eritematoso sistémico (LES). A pesar de considerarse un tratamiento seguro, existen efectos adversos descritos. Uno muy infrecuente es la trombocitopenia. Para establecer el diagnóstico de trombocitopenia causada por hidroxicloroquina es necesario descartar otras causas no farmacológicas y objetivar la recurrencia tras la reexposición al fármaco. Presentamos un caso de trombocitopenia severa en un paciente con LES durante el tratamiento con hidroxicloroquina.
The potential causes of thrombocytopenia are numerous and, depending on the mechanism of action, can be classified into 2 large groups: (1) immune-mediated—secondary to autoimmune diseases, idiopathic thrombocytopenic purpura, infections, immunodeficiencies and drug-induced; and (2) nonimmune-mediated—drug-induced, bone marrow diseases and congenital.1
Case ReportThe patient was a 27-year-old man who had been diagnosed with systemic lupus erythematosus (SLE) 5 years earlier, as a result of an episode of immune thrombocytopenia and primary peritonitis. The diagnosis revealed antinuclear antibodies (ANA) at a titer of 1:320, negative results for anti-double strand (ds) DNA antibodies, normal levels of complement component C3 and C4, and a systemic lupus erythematosus disease activity index (SLEDAI) of 11. Despite treatment with immunosuppressive agents and maintenance with mycophenolate sodium (360mg/12h) and hydroxychloroquine (200mg/day), the patient developed class II lupus nephritis with an activity index of 1/12 and of chronicity of 0/12. Renal biopsy was performed in June 2011: light microscopic study of 10 glomeruli showed moderate focal and segmental mesangial hypercellularity, although there were no lesions indicative of necrotic activity, karyorrhexis, crescents or fuchsinophilic deposits. Cellularity was preserved and there was no tubulointerstitial fibrosis or atrophy. Immunofluorescence of 4 glomeruli was positive, with a pure mesangial glomerular pattern. Some paramesangial deposits were focally and segmentally isolated. There was no evidence of parietal deposits. Tests revealed IgG−/+, IgA++, C3++, C1q−/+, Kappa+/+ and Lambda+/+. Negative findings in vessels, interstitium and tubules.
He had a proteinuria of 5g/day detected in May 2015 and was admitted to the hospital for another renal biopsy. During his hospital stay, he was found to have asymptomatic thrombocytopenia, 4×109cells/L, with no signs of cellular atypia, negative results on tests for antiplatelet and antihistone antibodies and a SLEDAI of 9. He began to take corticosteroids at a dose of 1mg/kg body weight (bw)/day, with a diagnosis of autoimmune thrombocytopenia. Immunosuppressive therapy was maintained, and hydroxychloroquine, which was not prescribed, was discontinued. After 48h, he had a rapid platelet recovery, reaching 195×109cells/L. This enabled the performance of the renal biopsy, which revealed a progression of the lupus nephritis, from class II to class V (membranous glomerulonephritis). Renal biopsy was repeated in July 2015. Light microscopic study ruled out the presence of lesions indicative of proliferative glomerulonephritis; there was no tubulointerstitial fibrosis and immunofluorescence of 5 glomeruli showed a generalized, diffuse glomerular parietal pattern: IgG+++, IgA++, IgM+/−, C3+++, C1q+, Kappa+++ and Lambda+++. Findings in vessels, interstitium and tubules were negative.
At discharge, a different physician again prescribed combined therapy with mycophenolate and hydroxychloroquine (200mg/day). The patient was readmitted 72h after discharge due to a new episode of severe thrombocytopenia, with no signs of lupus activity (negative test for anti-dsDNA antibodies and normal C3 and C4 levels). He began to take corticosteroids again (1mg/kg bw/day), without platelet recovery.
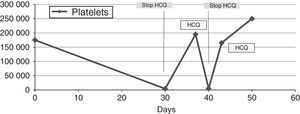
Given the rapid cause-and-effect relationship between hydroxychloroquine and thrombocytopenia, the drug was discontinued intentionally. Within 72h, the platelet count reached 200×109cells/L (Table 1).
Platelet Count, White Blood Cells, Red Blood Cells, Anti-DNA and Complement According to Exposure to and Interruption of Hydroxychloroquine (HCQ).
| Platelets | Hemoglobin | Leukocytes | Lymphocytes | Anti-DNA | C3 | C4 | |
|---|---|---|---|---|---|---|---|
| Baseline | 175×109/L | 141g/L | 9.2×109/L | 1.6×109/L | Negative | 0.9 | 0.15 |
| HCQ | 4×109/L | 140g/L | 9.4×109/L | 2×109/L | Negative | 1.3 | 0.13 |
| STOP HCQ | 195×109/L | 154g/L | 8.8×109/L | 1.7×109/L | Negative | 1.5 | 0.22 |
| HCQ | 5×109/L | 153g/L | 10×109/L | 1.8×109/L | Negative | 1.1 | 0.19 |
| STOP HCQ | 250×109/L | 148g/L | 9.5×109/L | 1.5×109/L | Negative | 1.0 | 0.2 |
During follow-up, after 1 month, the total platelet count was 250×109cells/L and, in analyses performed 3 and 6 months later, there had been no decrease (Fig. 1). He began to take tacrolimus because of the progression of nephritis.
DiscussionDrug-induced thrombocytopenia is based on the accelerated destruction of platelets that can occur via 2 different mechanisms: due to direct toxicity acting on the bone marrow or due to peripheral destruction by means of a mechanism that may or may not be immune-mediated by specific antibodies that react with the drug.2,3
The drugs that have been most frequently related to the development of thrombocytopenia are cinchona alkaloids (quinine/quinidine), sulfonamides, nonsteroidal anti-inflammatory drugs, diuretics, anticonvulsants, tuberculostatic drugs and heparins.3,4
The diagnosis of drug-induced thrombocytopenia is established by exclusion, by interrupting administration of the drug and by the detection of drug-dependent antibodies. The temporal relationship between the administration of the treatment and the development of thrombocytopenia is highly important, as is recurrence after re-exposure to the drug.4,5 In our patient, the discontinuance of the medication was used as a method of exclusion.
Reaching the definitive diagnosis in the case we report was complex, given that the first manifestation of SLE in our patient was thrombocytopenia. There is a form of clinical presentation in SLE consisting of chronic thrombocytopenia with a slight response to corticosteroids.6 Thus, the initial diagnostic suspicion focused on the exacerbation of the lupus activity, in a patient in whom, moreover, the disease had progressed to renal involvement.
Hydroxychloroquine is an antimalarial drug employed in the treatment of lupus and other rheumatic diseases. A number of retrospective studies have shown the efficacy of hydroxychloroquine in the treatment of the autoimmune thrombocytopenia associated with SLE. The authors recommend that the medication not to be discontinued, even in advanced stages of lupus nephritis. Nevertheless, the secondary effects of that drug include thrombocytopenia,7 although it is not a common adverse effect, given that there are only isolated cases reported in the literature.8–10
ConclusionFinally, we consider this case to be of clinical and practical interest, because the cause of the thrombocytopenia was related to hydroxychloroquine and not to the lupus activity, which at all times was clinically and serologically inactive, despite the severity of the thrombocytopenia.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Vázquez VA, Pascual L, Corominas Macias H, Giménez Torrecilla I. Trombocitopenia recurrente inducida por hidroxicloroquina en ausencia de actividad del lupus eritematoso sistémico. Reumatol Clin. 2017;13:294–296.