The first experiences with a group of drugs called immune checkpoint inhibitors for the treatment of cancer were described in 2010. They are currently used in many tumours, with successful survival outcomes but a new profile of adverse events. This new spectrum of immune-mediated toxicities includes an exaggerated inflammatory response of T lymphocyte and the development of autoimmune diseases or similar pathologies. Of these, of particular note are the rheumatological toxicities. This review aims to alert internists and rheumatologists to their recognition and clinical management.
En 2010 se conocieron las primeras experiencias con un grupo de medicamentos denominados inhibidores de los puntos de control inmunitario (IPCI) para el tratamiento del cáncer. Actualmente, se utilizan en diferentes tumores y estadios, modificando la sobrevida de los pacientes, pero generando un nuevo perfil de toxicidad. Este nuevo espectro de toxicidades inmunomediadas (irAE) son generadas por una exagerada respuesta inflamatoria de linfocitos T que puede desarrollar enfermedades autoinmunes o patologías similares. Entre ellas, se hallan las toxicidades reumatológicas. Esta revisión se propone alertar a los internistas y reumatólogos a reconocer las irAE reumatológicas y conocer su manejo clínico.
In 2010 the first experiences with a group of drugs known as immune checkpoint inhibitors (ICPI) for the treatment of cancer were published. ICPI are monoclonal antibodies against a specific group of proteins which are found on the T lymphocyte cellular membrane or their ligands: protein 4 of the cytotoxic lymphocyte antigen (CTLA4), programmed cellular death protein (PD1) and its ligand (PDL1); they function by blocking inhibitory T lymphocyte signals during the inflammatory response, these signals being necessary to exert immune tolerance. They thereby generate persistence of T lymphocyte inflammatory activity and thus strengthen the antitumour immune response, preventing the evasion of the immune system which tumour cells use to prevail1,2.
The ICPI in use now are: ipilimumab (anti CTLA4), nivolumab, pembrolizumab and cemiplimab (anti PD1) and atezolizumab, avelumab and durvalumab (anti PDL1). They have been approved for an increasingly wide spectrum of oncological diseases (melanoma, non-small cell lung cancer, kidney cell carcinoma, Hodgkin’s lymphoma, mesothelioma, urothelial tumours, tumours in the head and neck, triple negative breast cancer and merkelomas)3.
Although the ICPI have modified the prognosis of cancer patients, they have given rise to a new profile of toxicities, grouped under the name of immune-related Adverse Events [irAE]) which are due to the non-specific action of T cells. The clinical manifestations of the irAE are diverse as is their severity and the time they take to appear following exposure. This scenario means there is a need to integrate different specialities, including rheumatologists, when symptoms appear in the joints, muscles and bones which are very similar to those of systemic autoimmune diseases4.
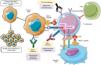
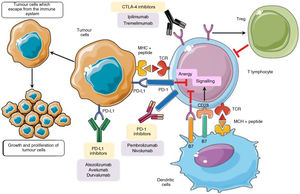
The physiopathology of irAECellular checkpoints exist in the physiological inflammatory response. These regulatory systems restrict the activation of T cells, preventing an immune hyper-response, the loss of homeostasis and immunological tolerance. T cell activation requires the binding of its receptor to an antigen, together with a second signal known as the co-stimulator which arises from the binding of CD28 (the T receptor accessory molecule) with the CD80/CD86 molecules that are present in the antigen-presenting cell, producing a positive signal and activating a cascade of secondary messengers which culminates in the start of the inflammatory response. CTLA4 is a molecule that is present in T lymphocytes. It has inhibitory characteristics and is homologous to CD28, with a high affinity for CD80/CD86, competing to bind with this molecule. When it does so, it prevents the generation of the second signal, thereby hindering activation of the T cell5,6. In a similar way, PD1 (programmed cellular death protein) is a negative regulator of T lymphocyte activity when it binds with their PDL1 and PDL2 ligands6.
CTLA4 expresses its activity early in the lymphatic ganglia and the thymus7, while T cell inhibition by PDL1 occurs in the later stages of the immune response8. PD1 and PDL1 play a key role in maintaining peripheral tolerance, controlling autoreactive T lymphocytes which have escaped from the central tolerance mechanism of the thymus2, and in situations where activated T cells are unable to eliminate the antigen, as occur in chronic infections or in the context of tumours9 (Fig. 1).
In the inflammatory response the B7 ligands expressed in the APC bind to the CD28 receptor in CTL, leading to T cell amplification and the immune response. Alternatively, the binding of B7 ligands to CTLA-4 expressed in T cells suppresses their activity. CTLA-4 also improves Tregs activity which leads to immunosuppressant activity. PD-1 is expressed in activated T cells. PD-1 binds to its PD-L1, which triggers CTL anergy and promotes the inhibitory signals even more. The pharmacological inhibition of the immune checkpoints with monoclonal antibodies restores inflammatory activity, including antitumour activity9.
CTL: cytotoxic T lymphocytes; CTLA-4: cytotoxic T lymphocyte antigen 4; DC: dendritic cell; MHC: major histocompatibility complex; PD-1: programmed cellular death -1; PD-L1: programmed cellular death ligand -1; TCR: T cell receptor; Tregs: regulatory T cells.
Adapted from Taieb et al.9.
When immune system activity increases the ICPI generate numerous immunomediated adverse effects (irAE) in up to 80% of the patients treated8. The main organs affected are the skin (maculopapular rash, eczema, pruritus), the gastrointestinal tract (colitis), the endocrine glands (hypothyroidism or hyperthyroidism, hypophysitis, suprarenal failure) and the liver (autoimmune hepatitis)10. The central nervous system is affected to a less degree (Guillain-Barré, encephalitis and transverse myelitis), as are the cardiovascular system (myocarditis), the lungs (pneumonitis), the haematological system, the joints and the musculoskeletal system7,8,11. This review covers the latter.
It is still a controversial question whether the appearance of an irAE predicts a favourable antitumour response. It has been suggested that the appearance of vitiligo or granulomatous skin lesions in patients with melanoma may be markers of a stronger therapeutic response12. In an Australian series of 244 patients treated with ICPI, 19 who developed rheumatological irAE had a good antitumour response13. Nevertheless, prospective studies are required to reach a conclusion on this point7,8,14.
General considerations about rheumatological irAEThe clinical presentation of rheumatological irAE is usually similar to rheumatological disease that is not ICPI mediated, although it is distinguished by its age and sex distribution. Antibodies are usually negative and few patients fulfil the classification criteria for autoimmune diseases.
Arthralgia is the most common symptom (10%–15% of patients), followed by myalgias and dry syndrome. A systematic review found that arthralgia occurred with a frequency of from 1% to 43% and myalgias in from 2% to 20% of cases treated with ICPI15. This lack of heterogeneity in reporting is due to the fact that patients are not referred to the rheumatologist for mild symptoms, or the fact that the presence of another concomitant irAE may be treated with immunosuppressant drugs by another specialist or the oncologist12,13,16.
From 3.5% to 6.6% of patients treated using ICPI require the intervention of a rheumatologist due to inflammatory arthritis, myositis, dry syndrome or vasculitis17,18.
Rheumatological irAE mechanismsirAE are caused by different mechanisms: 1) increased T lymphocyte activity against antigens expressed in the tumour as well as in healthy tissue; 2) increased titration of pre-existing antibodies due to the increase in B lymphocyte activity; 3) an increase in proinflammatory cytokines, complement mediated inflammation8.
The rheumatological irAE include inflammatory arthritis, myositis, rheumatic polymyalgia, psoriatic arthritis and reactive arthritis7,19,20. Vasculitis has also been reported, as have dry syndrome, remitting seronegative symmetrical synovitis with pitting oedema (RS3PE), tenosynovitis, enthesitis, reactions sclerodermiform reactions and sarcoidosis21.
Many patients simultaneously have more than one adverse immunological effect: colitis is the irAE that most often occurs concomitantly with arthritis, which is also associated with pneumonitis and thyroiditis12.
Although there is no predictive biomarker for the development of rheumatological irAE14, the development of any other irAE is considered to be a risk factor for the development of rheumatological symptoms22. In the following paragraphs we review the rheumatological findings which are most commonly associated with ICPI.
We should bear in mind that generalised musculoskeletal symptoms (arthralgia, myalgia or weakness) may also be associated with the underlying oncological disease, pre-existing arthritis, infection or the adverse effects of other drugs, all of which should be taken into account in differential diagnosis. Furthermore, acute phase reactants may be increased by the oncological disease or other irAE7.
Clinical manifestations of rheumatological irAEArthritisThis may arise in the following forms: symmetrical small joint arthritis, predominantly in the hands (similar to rheumatoid arthritis [RA]), asymmetrical oligoarthritis of large joints with predominant involvement of the knees (similar to spondyloarthritis) or compromise of the scapular and pelvic waist similar to rheumatic polymyalgia (RPM)6. Cases of reactive arthritis and psoriatic arthritis have also been described7,14. A major difference in comparison with classic rheumatological diseases is that these have no predilection for a specific sex, as there is a similar number of cases in men and women7,14. These cases are also usually negative for rheumatoid factor and citrulinated cyclic antipeptide (anti-PCC). They are more prevalent than enthesitis and tenosynovitis, and they require higher doses of corticoids than are habitually used for cases of inflammatory arthritis7. Only 20% of patients fulfil the classification criteria for RA or RPM21.
Diagnosis is clinical and is supported by imaging techniques23,24. The erythrocyte sedimentation rate and protein C reactive (CRP) are high, although the rise in these markers may also be due to the neoplasia.
The time from exposure to the drug until the appearance of inflammatory arthritis varies, with an average of 120 days21. Cases have been diagnosed after the suspension of ICPI, and cases have even evolved to become chronic in spite of the treatment having been suspended7,12,19,21,22. Functional incapacity, having received combined therapy with ICPI, being positive for FR and/or anti-PCC, and the finding of erosions could justify a more aggressive therapeutic approach25.
A systematic review by Ghosh et al.26 which included 372 patients, found that 49% had polyarthritis similar to RA and 17% had oligoarthritis. The authors suggest that this similar relationship between RA frequency and spondyloarthritis in the general population is because ICPI may induce these diseases in genetically predisposed patients. In fact, patients with ICPI-associated arthritis had a higher frequency of a shared epitope sequence in HLA-DRB1 compared with the general population, and this as we know is associated with the physiopathogenesis of RA27. A similar syndrome to RPM is found in 2% of the patients who receive ICPI. 25% of them do not fulfil the 2012 preliminary criteria of RPM of the European Alliance of Associations for Rheumatology/the American College of Rheumatology (EULAR/ACR). Some atypical characteristics were observed, such as joint involvement (chiefly the knees and hands), the absence of raised inflammatory markers, and aggressive cases that do not respond to the usual treatment. In these cases, symptoms should be investigated which suggest giant cell arthritis, which may often coexist22.
MyositisMyositis is a rare but potentially fatal adverse effect21. Although it may be hard to differentiate between paraneoplastic myositis or one induced by ICPI, painstaking questioning may detect the moment the symptoms started and its relationship with the commencement of treatment28. Prospective studies report only a few cases of ICPI-induced myositis, indicating a low prevalence of the same29,30. Nevertheless, it seems to be becoming increasingly common, probably due to the increasing number of patients treated using immunotherapy. ICPI-induced myositis has atypical characteristics in comparison with the idiopathic forms of the disease, and it gives rise to a high risk of mortality.
Myositis generally occurs after the first or second dose of ICPI, after an average of 25 days7,21. The main symptoms are proximal weakness and myalgia in the absence of the rash which characterises dermatomyositis; palpebral ptosis and diplopia should lead to the suspicion of concomitant myasthenia gravis. This is associated with myositis in up to 12.5% of cases, and it makes it necessary to explore bulbar symptoms and respiratory failure31. It usually involves raised CPK14,21. Diagnosis is based on physical examination and high levels of muscle enzymes, and eventually an electromyogram and magnetic resonance imaging of the muscles are required. The antibodies for inflammatory myopathies and antibodies against the acetylcholine receptor (AChR) are usually negative7,21. The role of muscle biopsy here is unclear; there are few reports of findings which reveal necrosis and inflammation, confirming harm to the muscle.
Myocarditis is in the same clinical spectrum as myositis22; cardiac assessment should include the search for clinical signs such as precordial pain, dyspnoea and conduction disorders. Troponins should be measured and a Doppler echocardiogram and electrocardiogram should be requested21. Signs of inflammation in cardiac magnetic resonance imaging have been reported in more than 60% of the patients with ICPI-induced myositis32.
Sarcoidosis and sarcoid-type reactionsThere are descriptions in the literature of cases of ICPI-induced sarcoidosis or sarcoid-type reactions, the majority of which occurred after monotherapy with anti-CTLA4 or anti-PD133. Although the pathogenic mechanism of ICPI-induced sarcoidosis is not fully understood, in idiopathic sarcoidosis there is a fall in CTLA4 expression in T-regulatory cells (T-reg) and Th17. This may lead to a defective T-reg suppressor function and, on the other hand, greater activation of Th17 cells, both of these being events with pathogenic implications25. This may explain, at least partially, the appearance of sarcoidosis after blocking CTLA4.
ICPI-induced sarcoidosis may present as cutaneous34 or systemic sarcoidosis, with lymphadenopathy and pulmonary compromise or neurological and ocular involvement35. There are no specific findings in serum for ICPI-induced sarcoidosis. Melanoma is the tumour most often associated with ICPI-induced sarcoidosis, and it varies in its moment of appearance, taking up to 200 days to appear after the start of treatment36.
This presentation is important in clinical and prognostic terms, as sarcoid-type reactions may be erroneously diagnosed as disease progression. The majority of the lymphatic ganglia biopsies performed in these cases to exclude the recurrence or progression of the cancer show non-necrosing granulomatous inflammation36.
Systemic vasculitisThere is a link between the physiopathology of idiopathic systemic vasculitis and the faulty expression of molecules which form part of the physiological immune control checkpoints, with less expression of the PD1/PDL1 pathway. In the hypothesis that vascular inflammation is closely associated with dysfunctional immune control checkpoints, it is not surprising that the inhibition of these checkpoints within the context of immunotherapy may cause vasculitis37. Cases have been reported of vasculitis in small, medium and large vessels7. A systematic review of 53 cases with the suspicion of vasculitis found that 20 were confirmed. The most frequent types were: large vessel vasculitis (giant cell arteritis and isolated aortitis) and vasculitis of the nervous system (central and peripheral). The average time to the appearance of symptoms was 3 months following the start of immunotherapy, and in the majority of cases under treatment with anti-PD138. This finding is concordant with studies that suggest a deficiency in the PD1/PDL1 pathway in the pathogenesis of large vessel vasculitis38. Cases have also been published of vasculitis with involvement of the retina or uterus, as well as one case of granulomatosis with polyangiitis with involvement of the lung and kidney14. One case of giant cell arteritis after the infusion of nivolumab presented with a large ulcer on the scalp31. Systemic vasculitis is know to be able to present severe clinical manifestations with damage to organs, so that if vasculitis is suspected ICPI should be suspended and steroid therapy commenced.
Dry syndromeImbalances in the immune checkpoints may induce the activation and proliferation of autoreactive cells which leads to dry syndrome39. CTLA4 polymorphisms have been correlated with susceptibility to the disease and the production of antibodies40.
Dry syndrome in patients treated with ICPI generally presents in acute form, without parotiditis and in the absence of anti-Ro and anti-La14. In a series of 4 cases, it presented as dry mouth with a sudden onset and signs of severe glandular hypofunctioning12. A recent review identified 17 cases of dry syndrome after treatment with ICPI41. These patients had a median age of 63 years, with a slight predominance of men (53%). The majority had melanoma (71%) and they received anti-PDL1 therapy (88%). The median time to appearance of the syndrome after the start of treatment was 3.8 months, and they had none of the typical antibodies of the disease.
Saliva gland biopsy showed non-Sjögren sialadenitis, with diffuse lymphocytic infiltration of T cells and acinar lesion mediated by the infiltration of CD4+ and CD8+ T cells25. In Sjögren sialadenitis is focal, with dense aggregates of 50 or more lymphocytes plus perivascular or periductal lymphocytes adjacent to mucosal acini with a normal appearance42.
Systemic lupus erythematosusThe deregulation of immune checkpoints is involved in the pathogenesis of systemic lupus erythematosus (SLE). Data suggest that polymorphisms in the PD1 and CTLA4 genes in humans and animal models may cause manifestations of lupus43. Although SLE is the prototype systemic autoimmune disease, ICPI-induced lupus is very rare44. According to the adverse event notification system of the Food and Drug Administration (FDA), until June 2018 only 18 cases of SLE had been reported, with 7 cases of cutaneous lupus, 2 of a syndrome similar to lupus, one case of lupus nephritis and one of central nervous system lupus associated with ICPI45. It presents late, with an average of more than 120 days after the start of ICPI; the age group (60 years) is different from that of classic lupus, and there is a predominance of men.
Sclerodermiform syndromesTwo cases have been reported of scleroderma in patients under treatment with pembrolizumab for melanoma: one of them had localized scleroderma and the other with diffuse scleroderma, both of which were confirmed by biopsy of fibrosis and cutaneous sclerosis33. Although the underlying mechanism is unknown, it is suggested that anti-PD1-mediated inflammation may lead to activation of β (TGF-β) transforming growth factor, triggering a profibrotic cascade46.
Thirteen cases of eosinophilic fasciitis were found in the literature. The majority of these cases were patients treated for melanoma with anti-PD1 therapy47.
Crystal arthritisOne case has been described of recurring attacks of pseudo-gout in the knee joint of a patients treated with nivolumab for kidney cancer, 7–10 days after each infusion; these events ceased under prophylaxis with colchicine. Analysis of the synovial fluid from the said patient found calcium pyrophosphate crystals and an increase in IL-17 which correlated with the increase in synovial neutrophils. The authors suggest a potential effect of Th17 on the recruitment of neutrophils in pseudo-gout events induced by ICPI48.
Bone involvementA warning has been issued about irAE with involvement of the bone. 6 cases have been reported with two different phenotypes of skeletal involvement: 3 patients had new non-traumatic spinal fractures (one of them with multiple fractures, including the ribs and pelvis) and 3 with localized bone resorption. Five of these cases were in men. None of the patients with fractures had osteoporosis detected by densitometry, and none of them had any additional risk factors for osteoporosis49.
Antiphospholipid syndromeOne case in the Ambroise Paré University Hospital (Belgium) reports a patient with lung cancer treated with pembrolizumab who suffered a cerebrovascular accident in the territory of the right medial cerebral artery, with IgG anti-cardiolipin and IgG positive anti-B2 glycoprotein 1 IgG positive antibodies. The authors reviewed the literature and found 4 similar cases in patients with melanoma, including one case of catastrophic antiphospholipid syndrome50.
Treatment of rheumatological irAEThe irAE have classically be classified according to severity in 5 different categories (from 1 for mild toxicity up to 5 for mortality); each category is treated according to its clinical impact, defining the continuity, suspension and/or re-exposure to the treatment with ICPI. In turn, and according to the category, diagnostic studies are recommended as well as referral to rheumatology. Different guides have been published with recommendations for the management of toxicities: the most important of these are the one by the European Society of Medical Oncology (ESMO), 201751, the American Society of Clinical Oncology (ASCO), 201952, and the National Comprehensive Cancer Network (NCCN) guide, which was updated most recently in February 202253. All of these guides contain similar recommendations.
In 2020 the EULAR guides for the management and diagnosis of rheumatological irAE caused by ICPI were published21, underlying the importance of including a rheumatologist in the team working on the management of these patients. The therapeutic recommendations are summarised below:
- •
If symptomatic treatment is not effective, use local or systemic corticoids. The dose and form of administration depend on the symptoms and degree of activity. When an improvement in the symptoms is achieved, a gradual reduction of systemic corticoids is suggested, down to the minimum effective dose.
- •
Classic disease modifying antirheumatic drugs (DMARD) should be considered when the response to corticoids is insufficient, or to reduce the use of corticoids.
- •
When patients have severe manifestations or insufficient response to classic DMARD, then biological DMARD should be considered, of which the anti-TNF or IL-6 inhibitors are the options of choice for inflammatory arthritis.
- •
Myositis is a severe condition. In the presence of life-threatening symptoms, such as bulbar symptoms (dysphagia, dysarthria or dysphonia), dyspnoea or myocarditis, then high doses of corticoids, human immunoglobulin or plasma exchange should be considered. Immunotherapy should always be suspended in such cases.
- •
Before the start of immunotherapy autoantibody doses are not indicated in all patients. If there are rheumatological or musculoskeletal manifestations or previous systemic symptoms then a complete rheumatological evaluation should be undertaken.
It is not known whether the use of steroids may modify the anti-tumour response; retrospective studies have shown that the outcomes were similar in those patients who received immunosuppressants for the irAE8,54. Some authors suggest that minimizing the exposure to steroids may improve control of a tumour over the long term, while also limiting adverse metabolic events55. We therefore propose a change in the treatment paradigm for rheumatological irAE, from the conventional model in which corticoids are at the base of the pyramid, which progresses upwards to classic and then biological DMARD, to an inverted pyramid, where the use of anti-cytokine treatments or intracellular signalling inhibition is prioritized, reducing the use of corticoids6.
The appearance of an irAE led to the definitive suspension of the ICPI in 38% of patients, and a temporary suspension in 11%, according to a systematic review26. According to the Clinical Practice Guides of the Society for Immuno Therapy of Cancer Treatment (SICT), the patients who experience grade 2 irAE may be re-exposed to the drug if the signs and symptoms resolved or are controlled by less than 10 mg/day prednisone or equivalent. The decision to re-expose the patients who develop grade 3 or 4 irAE should be taken on an individual basis, depending on the potential risks and benefits of the therapy. Patients with myositis who experience myocarditis should permanently interrupt the therapy56.
Immune networks are complex, and different monoclonal antibodies, immunosuppressants and immune signalling inhibitors may affect the efficacy of the ICPI in different ways56,57. When commencing immunosuppressant treatment due to an irAE, it is therefore necessary to insist on using the smallest possible dose to achieve a therapeutic effect.
Use of immunotherapy against cancer in patients with previous autoimmune diseasesPatients with autoimmune diseases were excluded from the clinical trials of immunotherapy for cancer7,14. Nevertheless, there are now series of cases which support the use of immunotherapy in patients with pre-existing rheumatological diseases.
A systematic review which included 123 patients with pre-existing autoimmune diseases found that 41% underwent aggravation of their underlying disease, 25% had irAE (the most frequent of which were colitis and hypophysis) and 9% had both complications58. No significant difference was found in terms of irAE between those who had active or inactive autoimmune disease. The patients who were receiving immunosuppressant treatment prior to immunotherapy had fewer irAE (59%) than did those without treatment (83%). 62% of the patients required high doses of corticoids for treatment and 16% required DMARD or other immunosuppressants. The irAE improved in 90% of the patients. Cappelli and Bingham57 reported that up to 40%–50% of patients with autoimmune diseases who received ICPI may develop aggravation of their underlying disease, mainly RA and psoriatic arthritis; there are no reliable data for vasculitis, scleroderma or SLE.
It may be concluded that the use of ICPI in patients with previous autoimmune and inflammatory diseases is based on balancing the risk and benefit for each individual. Although the treatment often triggers an exacerbation of the previous disease, on the other hand the appearance of irAE is associated with a better therapeutic response, according to the recent observation in a study by the Memorial Sloan Kettering Cancer Center59.
ConclusionsIt cannot be denied that ICPI therapy is now a part of cancer treatment, generating a toxicity profile that obliges us to work in an interdisciplinary way.
The clinical spectrum of rheumatological toxicities is multiple and not so infrequent. Toxicities occur more often with anti-PD1 therapy. Arthralgias are usually the first symptom, and symmetrical polyarthritis and rheumatic polymyalgia are the most common inflammatory symptoms. Due to their severity, inflammatory myopathies are the cause of extreme alarm. We should take into account the fact that the clinical presentation may be atypical, usually with negative antibodies and a similar prevalence in men and women. To date there is no contraindication in commencing immunosuppressant therapy to treat them.
Delay in the diagnosis of a rheumatological irAE conditions the prognosis for these patients; rheumatological symptoms may persist even after the suspension of the treatment, and possible damage may lead to a permanent functional limitation. Table 1 shows the relationship between the tumour, the type of drug and the associated irAE.
Summary of the ICPI, mechanism of action, indications and rheumatological irAE.
| Mechanism of action | Drug | Indications | Adverse rheumatological effect |
|---|---|---|---|
| Anti CTLA4 | Ipilimumab | • Cutaneous and metastatic melanoma | Unspecified arthritis11,16 |
| • Advanced kidney carcinoma | Dry syndrome16 | ||
| • Metastatic colorectal cancer | Nephritis lupus32 | ||
| Vasculitis31 | |||
| Sarcoidosis37 | |||
| Tremelimumab | Used combined with durvalumab in the treatment of stage IV non-small cell lung carcinoma | ||
| Anti PD1 | Nivolumab | • Melanoma, non-small cell lung cancer | Unspecified arthritis13,16,25 |
| • Kidney carcinoma | Monoarthritis23 | ||
| • Hepatocellular carcinoma | Oligoarthritis23 | ||
| • Hodgkin’s lymphoma | PMR mimic13,14,20,23 | ||
| • Squamous cell carcinoma of the head and neck | RA mimic11,14 | ||
| • Urothelial carcinoma | JIA11 | ||
| • Colorectal cancer with microsatellite instability | Psoriatic arthritis11 | ||
| Arthritis caused by CPPD deposition25 | |||
| Pseudogout29 | |||
| Myositis11 | |||
| Dry syndrome13,16,23 | |||
| Vasculitis25 | |||
| Sarcoidosis36 | |||
| Spinal fracture41 | |||
| Bone resorption41 | |||
| Pembrolizumab | • Melanoma | Arthritis25 | |
| • Non-small cell lung cancer | Monoarthritis23 | ||
| • Hodgkin’s lymphoma | Oligoarthritis23 | ||
| • Squamous cell carcinoma of the head and neck | PMR mimic14,20 | ||
| • Urothelial carcinoma | RA mimic11,14 | ||
| • Stomach cancer | Olecranon bursitis25 | ||
| • Solid tumours with major microsatellite instability | Myositis11,23,30 | ||
| Sarcoidosis35 | |||
| Eosinophilic fasciitis34 | |||
| Vasculitis31 | |||
| APS40 | |||
| Spinal fracture41 | |||
| Anti PDL1 | Atezolizumab | • Metastatic non-small cell lung cancer | PMR mimic14,20 |
| • Urothelial carcinoma | Dry syndrome13 | ||
| Avelumab | • Metastatic Merckel cell carcinoma | ||
| • Locally advanced or metastatic urothelial carcinoma | |||
| Durvalumab | • Locally advanced or metastatic urothelial carcinoma | ||
| • Stage III unresectable non-small cell lung cancer | |||
| Combined therapy | Ipilimumab + Nivolumab | Metastatic or unresectable melanoma | Unspecified arthritis13,16 |
| Polyarthritis23 | |||
| Oligoarthritis23 | |||
| PMR mimic13,20,23 | |||
| Dry syndrome11,13,16 | |||
| Myalgias11 | |||
| Vasculitis31 | |||
| Sarcoidosis39 | |||
| Bone resorption41 | |||
| Ipilimumab + Pembolizumab | Unspecified arthritis13 | ||
| Monoarthritis23 | |||
| Tremelimumab + Durvalumab | • Stage IV non-small cell lung carcinoma | Myositis13 |
This review attempts to answer some of the questions raised by Nóvoa Medina and Rodríguez Abreu, who published three questions in REUMATOLOGÍA CLÍNICA on rheumatological irAE: 1) the real incidence of rheumatic pathology, taking into account the possible under-estimation due to the lack of standardization in the diagnosis; 2) the impact of treating the irAE on the tumour and in the new rheumatological disease, and 3) the concomitant effect of the immunotherapy and immunosuppression in patients with a history of rheumatological disease60.
Conflict of interestsThe authors have no conflict of interests to declare.