Clinical trials of secukinumab have demonstrated their efficacy and safety in psoriatic arthritis as biological first choice or after inadequate response to other biological treatments.
ObjectiveTo analyze the efficacy and safety of secukinumab in peripheral psoriatic arthritis over 12 months in real clinical practice.
Material and methodsPatients with active peripheral psoriatic arthritis who started treatment with secukinumab according to the technical specifications were included. Efficacy and safety were evaluated from baseline to 12 months comparing naive and non-naive to biological therapy patients.
Results76 patients were included (22 naive and 54 non-naive to biological) with an age of 51.9 years (10.3) and duration of the disease of 9.5 years (7.1). 31.6% with dactylitis, 51.3% with enthesitis and the baseline DAPSA was 19.0 (9.8). The retention rate was high, 90.9% in naive and 81.5% in non-naïve patients, and the percentage of patients with a DAPSA less than or equal to 14 was higher in the naive patients even after adjusting for age, sex and FAMEsc (p = .016). The safety data were similar to those described in the clinical trials.
ConclusionsSecukinumab is effective and safe in 12-month treatment in peripheral active PsA in real clinical practice, after inadequate response to TNF or as first biological treatment.
Los ensayos clínicos de secukinumab han demostrado su eficacia y seguridad en la artritis psoriásica como biológico de primera opción o tras respuesta inadecuada a otros tratamientos biológicos.
ObjetivoAnalizar la eficacia y seguridad de secukinumab en la artritis psoriásica periférica durante 12 meses en práctica clínica real.
Material y métodos: Se incluyeron pacientes con artritis psoriásica periférica activa que iniciaron tratamiento con secukinumab según ficha técnica. Se evaluó la eficacia y seguridad desde la basal hasta los 12 meses comparando pacientes naive y no naive a biológico.
ResultadosSe incluyeron 76 pacientes (22naive y 54 no naive a biológico) con una edad de 51,9 años (10,3) y duración de la enfermedad de 9,5 años (7,1). El 31.6% con dactilitis, 51.3% con entesitis y el DAPSA basal fue 19 (9,8). La tasa de retención fue elevada, 90,9 % en naive y 81,5 % en no naive, y el porcentaje de pacientes con un DAPSA menor o igual a 14 fue mayor en pacientes naive, incluso después de ajustar por edad, sexo y FAMEsc p = 0,016. Los datos de seguridad fueron similares a los descritos en los ensayos clínicos.
ConclusionesSecukinumab es eficaz y seguro en el tratamiento a 12 meses en la APs periférica activa en la práctica clínica real, tras respuesta inadecuada a los iTNF o como primer biológico.
Psoriatic arthritis (PsA) is a chronic inflammatory disease which chiefly affects the skin and locomotive apparatus, involving the peripheral and axial joints, enthesitis and dactylitis1. The European League Against Rheumatism as well as the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis have developed guides for the most suitable management and treatment of these patients.2,3 The treatment objective for all patients with PsA is considered to consist of achieving the lowest possible level of disease activity in all of its manifestations, minimising the complications caused by untreated active disease as well as those of the treatment used.4
In the first phase of treatment of the peripheral forms of PsA still considers the selection of using non-steroid anti-inflammatory drugs, corticoids (mainly local ones) and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD) such as methotrexate or leflunomide. When these do not achieve the therapeutic objective, the decision is taken to start a biological treatment that, until a few years ago, consisted above all of TNF inhibitors (TNFi). The biological treatments that are now available for PsA have focused on key cytokines for disease pathogenesis such as IL23 or IL17, with the emergence of drugs which aim to inhibit their effects.
Secukinumab is a human monoclonal antibody that blocks the effect of IL17A. In clinical trials it has been proven to offer a good clinical response in psoriatic arthritis as a first-line biological therapy or following an inadequate response to other biological treatments. It currently accounts for 25% of initial treatments for PsA with biological agents in the U.S.A.,5 indicating that rheumatologists may consider the use of secukinumab in the first stage of the biological treatment algorithm for the said disease. It has to be taken into account that, in the phase III controlled and randomised clinical trials with secukinumab, approximately 65–70% of the patients were naive to the TNFi.6–9
The results of the different clinical trials show that the patients treated with secukinumab achieve higher rates of remission maintained over time, mainly if they had not been treated beforehand with TNFi.10 It is also possible to achieve MDA in a higher percentage of these patients at both doses, 300 mg and 150 mg.11 Additionally, in FUTURE 5 it was found that remission according to DAPSA (Disease Activity index for Psoriatic Arthritis) occurred in 15.2% of the patients.9 The safety profile was similar in the different clinical trials with secukinumab, and with few differences respecting the placebo, with evidence that this was dose-dependent.
The purpose of this study was to analyse the clinical response and safety of secukinumab in actual clinical practice, in patients with PsA who had started treatment with it as their first biological drug or after having been treated with biological drugs beforehand.
Material and methodsThis is a multicentre (6 hospitals in Spain took part), observational, non-interventionist and descriptive study, carried out with patients who started treatment with secukinumab from 1 April 2017 to 1 April 2018. The patients who are analysed were aged ≥18 years old and fulfilled the CASPAR classification criteria for PsA.12 They had musculoskeletal involvement with moderate to high activity as measured by DAPSA13 in spite of treatment with csDMARD (methotrexate, leflunomide or sulfasalazine) and they were considered to be candidates for biological treatment according to the recommendations for the management of PsA.2,3,14 Patients were included who started treatment with secukinumab (with or without an associated DMARD) as their first biological drug, or after failed treatment with a previous biological drug, at a monthly dose of 150 mg or 300 mg, respectively, and with an induction dose, as recommended on the technical data sheet of the drug,15 at weeks 0, 1, 2, 3 and 4. Patients with purely axial involvement (without peripheral inflammation) were excluded, as were those whose main indication for treatment was axial or cutaneous activity. The patients were checked in rheumatology departments according to normal clinical practice, and disease activity was evaluated by joint count, the presence of enthesitis and dactylitis, as well as the DAPSA index and its cut-off points16 at the moment of starting treatment and 12 months after commencing treatment, or when the patient discontinued the treatment. Skin involvement measured by body surface area was classified as slight (<5%), moderate (5%–10%) or severe (>10%).17 Adverse events were recorded when they occurred during the follow-up period.
The results obtained by analysis of the qualitative or categorical variables are shown in the form of percentages and frequencies, and those corresponding to quantitative variables are shown as an average and standard deviation, after checking that they follow a normal distribution. Differences are considered to be clinically significant when P < .05. SPSS 17.0 software was used for all of the analyses.
All of the patients signed an informed consent document prior to starting treatment, to permit data gathering and analysis in an anonymous way, and they were included in a PsA patient registry (registry code: 2015/671).
Results76 patients with an average age of 51.9 years old (10.3) were included. The distribution of the sexes was 37 women (48.7%) and 39 men (51.3%), with a disease duration since diagnosis of 9.5 years (7.1). 24 patients had dactylitis (31.6%) and 39 has enthesitis (51.3%). The majority had a multiple-joint peripheral form of the disease, or mixed involvement (axial and peripheral), each with 32 patients (42.1%). The 12 remaining patients were peripheral oligoarticular forms (15.8%).
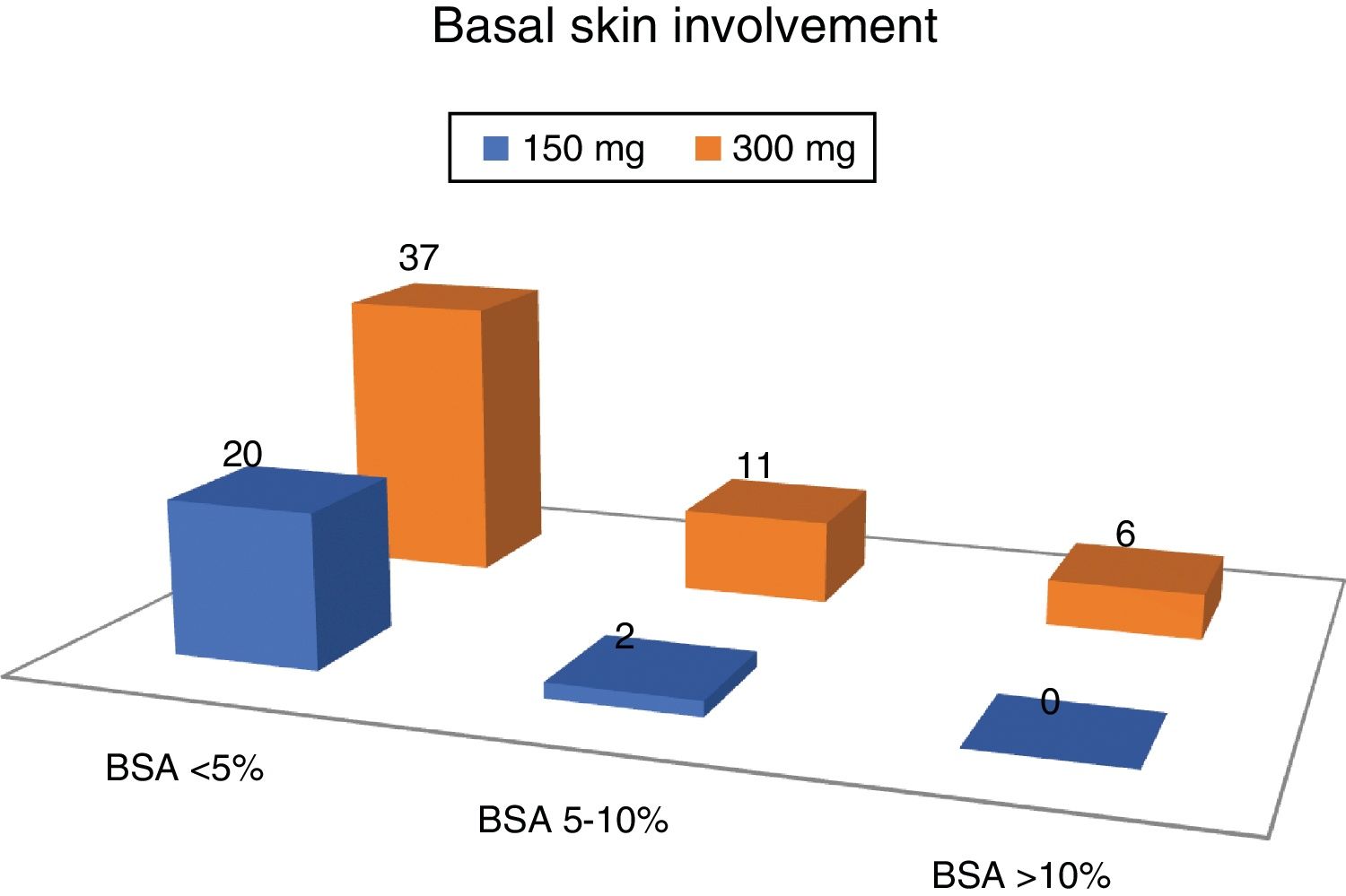
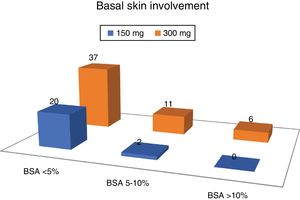
The average DAPSA at the start of treatment with secukinumab was 19.0 (9.8). Regarding cutaneous involvement, 8 patients (10.5%) had no psoriasis at the moment of commencing with secukinumab, 49 had slight involvement (64.5%), 13 moderate involvement (17.1%) and 6 severe involvement (7.9%).
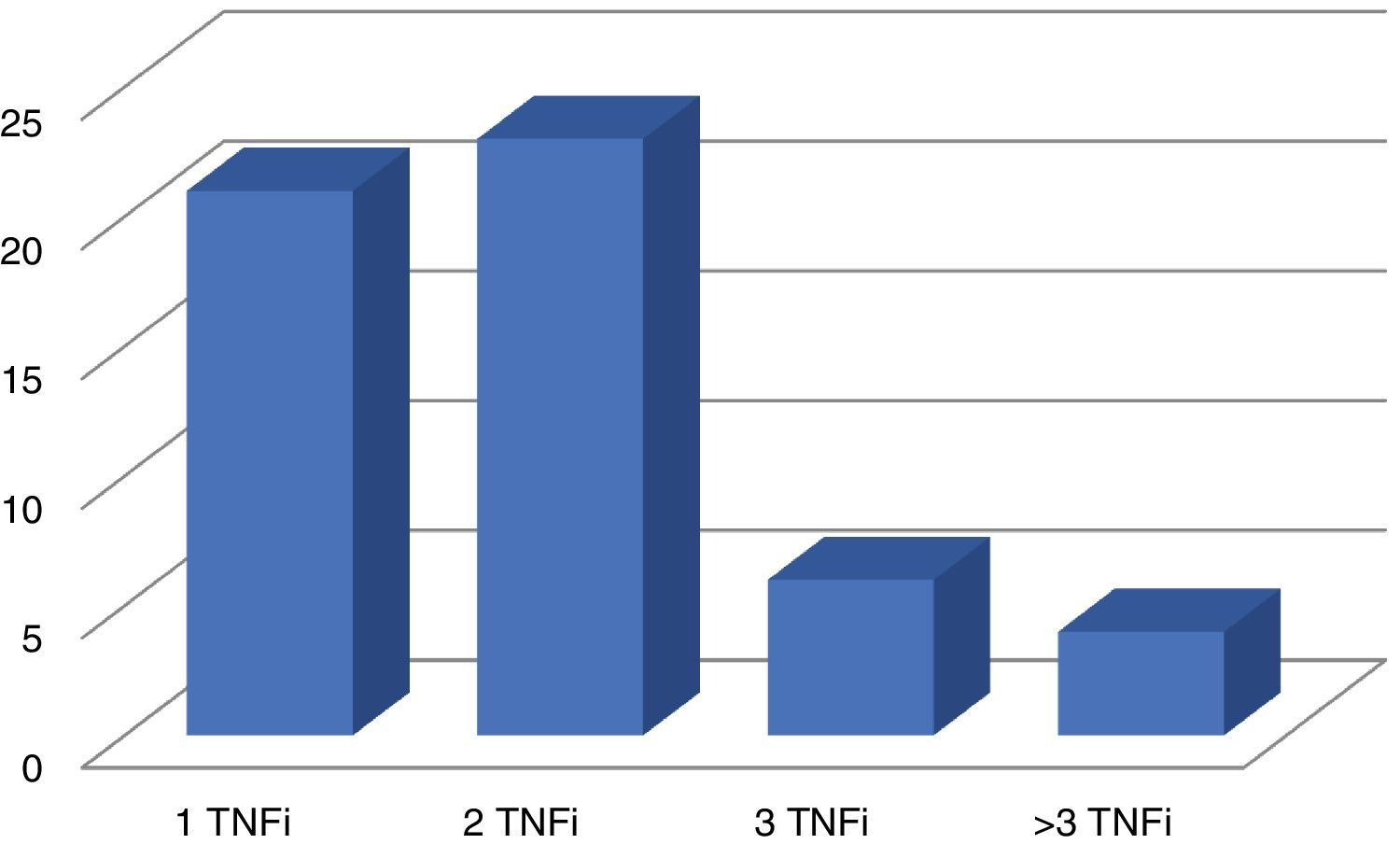
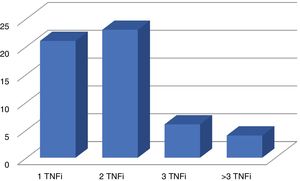
Disease activity was the reason why secukinumab was prescribed in 75 patients, and it was only prescribed in one patient due to the side effects associated with the previous treatment. 22 patients (28.9%) received it as their first biological treatment at a dose of 150 mg per month. The others, 54 patients (71.1%), received a dose of 300 mg per month, after having received at least one biological treatment beforehand: TNFi in 54 cases (Fig. 1) and ustekinumab in 2 patients. 59.2% (45 patients) received secukinumab without an associated csDMARD, while 27.6% also received methotrexate, 10.5% received leflunomide and 2.6% with sulfasalazine.
More than 2/3 of the patients had slight involvement of the skin, and no patient with severed skin involvement was taking the 150 mg dose of secukinumab (Fig. 2).
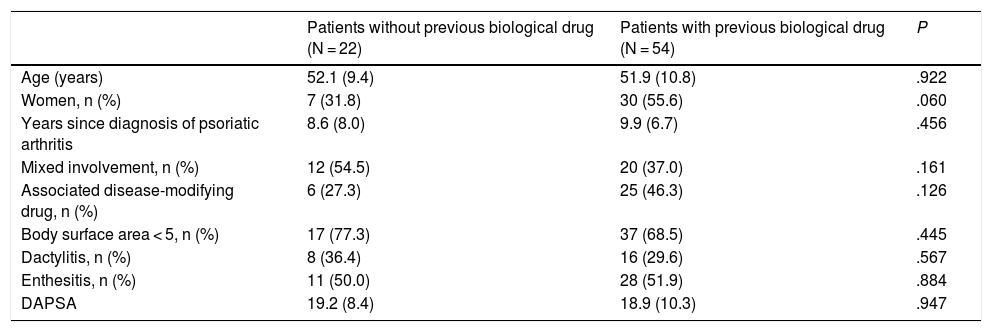
Table 1 shows the basal characteristics of the patients according to their dose of secukinumab, and Table 2 shows the clinical response and drug discontinuations. No significant differences were observed in the bivariate analysis between the variables analysed in the patients taking secukinumab 150 mg (without having previously taken a biological drug) and those taking 300 mg (after at least one biological treatment), except in the case of the patients who achieved low or very low disease activity as measured by DAPSA. Although in both groups of patients the retention rate at 12 months was high and with no statistically significant differences: 90.9% for the 150 mg group and 81.5% for the 300 mg group, the percentage of patients with a DAPSA score lower than or equal to 14 (VLDA and LDA) was higher in the group of naive patients (150 mg; P = .005). In the binary logistic regression analysis (taking into account age, sex, years with PsA, dactylitis and csDMARD) this difference was maintained, with an adjusted OR of 5.502 (CI 95%: 1.371–22.070; P = .016).
Basal characteristics of the patients treated with secukinumab (naive and not naive to biological drugs).
| Patients without previous biological drug (N = 22) | Patients with previous biological drug (N = 54) | P | |
|---|---|---|---|
| Age (years) | 52.1 (9.4) | 51.9 (10.8) | .922 |
| Women, n (%) | 7 (31.8) | 30 (55.6) | .060 |
| Years since diagnosis of psoriatic arthritis | 8.6 (8.0) | 9.9 (6.7) | .456 |
| Mixed involvement, n (%) | 12 (54.5) | 20 (37.0) | .161 |
| Associated disease-modifying drug, n (%) | 6 (27.3) | 25 (46.3) | .126 |
| Body surface area < 5, n (%) | 17 (77.3) | 37 (68.5) | .445 |
| Dactylitis, n (%) | 8 (36.4) | 16 (29.6) | .567 |
| Enthesitis, n (%) | 11 (50.0) | 28 (51.9) | .884 |
| DAPSA | 19.2 (8.4) | 18.9 (10.3) | .947 |
Results expressed as a percentage or mean (standard deviation).
Data on efficacy and safety over 12 months according to dose.
| Patients without previous biological drug (N = 22) | Patients with previous biological drug (N = 54) | P | |
|---|---|---|---|
| DAPSA VLDA/LDA, n (%) | 18 (81.8) | 25 (46.3) | .005 |
| Continued with secukinumab, n (%) | 20 (90.9) | 44 (81.5) | .491 |
| Primary failure, n (%) | 1 (4.5) | 7 (12.9) | .501 |
| Secondary failure, n (%) | 1 (4.5) | 3 (5.6) | .698 |
| Discontinued due to adverse effect, n (%) | 0 (0.0) | 1 (1.9) | .640 |
DAPSA VLDA/LDA: very low activity or low activity (<14).
2 cases of genitourinary candidiasis occurred (patients with diabetes), one in each treatment group, and they did not require the suspension of treatment. The discontinuation rate of secukinumab in the 12 months was low, and it was similar in both groups, and only one patient dropped out due to a severe adverse event in the 300 mg group, due to pneumonia.
DiscussionThe results obtained with these patients in actual clinical practice show that secukinumab obtains substantial clinical improvements in patients with active PsA, with high retention rates and safety data at 12 months that are similar to those observed in different clinical trials of the drug.
These data agree with the growing body of evidence which supports the use of IL17 inhibitors in PsA, and which is recognised internationally by different guides and recommendations for the treatment of PsA,2,3 including as the initial biological treatment after an inadequate response to csDMARD.
FUTURE 5 is the largest randomised phase III clinical trial of a biological drug for PsA,9 and in this it was observed that the subcutaneous administration of secukinumab at 300 mg and 150 mg brought about a rapid and significant improvement in clinical manifestations in comparison with the placebo, although secukinumab at 300 mg gave better responses than the 150 mg dose, with or without an induction dose, and in the final clinical objectives such as ACR20/ 50/70 or the resolution of enthesitis and dactylitis, particularly in naive patients to the TNFi.
The data from this study of actual clinical practice show a good response to the drug in biological therapy naive patients as well as in those treated beforehand with a biological drug, chiefly TNFi. Nevertheless, the best clinical responses were observed in patients who were naive to biological therapy, as a higher proportion of these patients attained a DAPSA showing low activity and remission than was the case for those previously treated with a TNFi. This finding agrees with the above-mentioned data from clinical trials.9
To date little information has been available regarding the efficacy and safety of secukinumab in actual clinical practice, and what we do know derives from different clinical trials, post hoc studies and survival data following commercialisation.18
The safety profile was consistent with what has already been published in the clinical trials of the drug.18,19 Thus the infections by Candida ―probably attributed to the role of IL17 in the defence of host mucus membranes against fungal infections ― observed in this study agree with the results of phase III studies of secukinumab (FUTURE 1–5): they were mild infections and did not require the suspension of the drug, simply needing management with the recommended antifungal therapy.19
Regarding the limitations of this study, we would firstly underline its observational nature. However, this characteristic may to a certain extent be a strength, as it permits observation of what occurs in patients with a certain treatment without interfering in any way, showing us the whole heterogeneity of the population studied. The small sample size is another limitation, so that the conclusions drawn from analysis of the same should also be accepted with precaution.
Taking into account the results obtained in this work, we are able to conclude that secukinumab is an effective and safe drug for the treatment of active peripheral PsA over 12 months in actual clinical practice. This is so for patients with previous experience of biological therapy as well as for those without this, and better results are even obtained in reducing inflammatory activity in the latter, with or without an associated csDMARD.
FinancingThis study received no specific grants from public, commercial or not-for-profit organisations.
Conflict of interestsJosé A. Pinto Tasende has received fees for congresses or scientific consultancy from BMS, Celgene, Janssen, Novartis, Pfizer and MSD.
We would like to thank the Sociedad Gallega de Reumatología.
Please cite this article as: Pinto Tasende JA, Maceiras Pan FJ, Mosquera Martínez JA, Dominguez LF, Rey BC, Porrúa CG. Secukinumab como tratamiento biológico en la artritis psoriásica en práctica clínica real. Reumatol Clin. 2021;17:203:206.