To provide a reference to rheumatologists and other physicians involved in the treatment of systemic lupus erythematosus (SLE) who are using, or about to use biologic therapies.
MethodsRecommendations were developed following a nominal group methodology and based on systematic reviews. The level of evidence and degree of recommendation were classified according to a model proposed by the Center for Evidence Based Medicine at Oxford. The level of agreement was established through a Delphi technique.
ResultsWe have produced recommendations on the use of belimumab, the only biological agent with approved indications for SLE, and other biological agents without an indication for SLE. The objective of treatment is to achieve a complete clinical response, taken as the absence of perceived or evident disease activity. Nuances regarding the use of biologic therapies in SLE were reviewed as well, such as the evaluation that should be performed prior to administration and the follow up of patients undergoing these therapies.
ConclusionsWe present the SER recommendations for the use of biological therapies in patients with SLE.
Elaborar unas recomendaciones que sirvan de referencia a los reumatólogos y otros profesionales implicados en el tratamiento del lupus eritematoso sistémico (LES) que vayan a utilizar o consideren la utilización de terapias biológicas en su manejo.
MétodosLas recomendaciones se emitieron siguiendo la metodología de grupos nominales y basadas en revisiones sistemáticas. El nivel de evidencia y el grado de recomendación se clasificaron según el modelo del Center for Evidence Based Medicine de Oxford y el grado de acuerdo se extrajo por técnica Delphi.
ResultadosSe realizan recomendaciones sobre el uso de belimumab, actualmente única terapia biológica con aprobación para el tratamiento del LES, y otras terapias biológicas sin indicación aprobada en LES. El objetivo del tratamiento es la respuesta clínica completa, entendida como la ausencia de actividad clínica percibida o constatable. Se matiza el uso de terapias biológicas en LES y cuál debe ser la evaluación previa y la vigilancia del paciente que recibe estos fármacos.
ConclusionesSe presentan las recomendaciones SER sobre el uso de terapias biológicas en el LES.
Systemic lupus erythematosus (SLE) is a very heterogeneous disease, both in its clinical manifestations as in its clinical course.1 It is more common in females and in certain races, and its peak incidence is between 15 and 40 years old. In Spain, the EPISER study estimated a prevalence of up to 91 cases per 100000 inhabitants.2
The treatment of SLE is conventionally based on the use of glucocorticoids, aspirin, nonsteroidal anti-inflammatory drugs, anti-malarials and various immunosuppressants. These treatments have significantly improved the prognosis of the disease in most patients, although not all respond and sometimes their use results in significant toxicity.
In recent years, biological therapies (BT) have been developed aimed at important specific targets in the pathogenesis of SLE.3 Belimumab (BLM) is the first specific BT with a specific indication for SLE in its technical insert4 but for other BT have also been used in spite of having an approved indication for other rheumatic diseases.5
All this led the Spanish Society of Rheumatology to generate a consensus on the use of BT in SLE, making recommendations based on the best available evidence and expert opinion. The goal is to help professionals who treat adult patients with SLE in relation to the use of BT: a) to reduce the variability in the use of BT in SLE, b) to bring the clinical practice closer to the best current scientific evidence, and ultimately, c) to support those involved in making decisions on the treatment of patients with SLE.
MethodologyThe Spanish Society of Rheumatology (SER) considered relevant to produce recommendations on the use of BT in SLE. To make this consensus we used a modification of the RAND/UCLA methodology.6 Nominal groups were created and Delphi surveys and systematic reviews of controversial topics performed.
We created a panel of experts in SLE using the following criteria: a) they had published articles on SLE, b) articles that were indexed in MEDLINE or published in Reumatología Clínica or the Spanish Journal of Rheumatology, and/or c) there was an interest and obvious experience in this field.
We conducted a nominal group meeting moderated by members of the Research Unit (RU) of the SER, in which decisions were made on the content and the sections of the document as well as its scope and intended audience. Based on the content determined, panelists raised questions that could be answered by systematic review.
In the first phase, each panelist was assigned to the development of one of the sections of consensus. Once all sections were consolidated into one document and edited by members of the RU, the full text was forwarded to all the panel for correction and generation of recommendations. Finally, we assessed the degree of agreement with the recommendations and drafted the final document. The degree of agreement was defined as the percentage of consensus among the panelists obtained from the vote for each recommendation through an anonymous survey. Consensus was considered when at least 70% of 7 or more panelists rated 7 on a scale from 1 (complete disagreement) to 10 (complete agreement). Those recommendations that did not reach a level of greater than 70% agreement were reformulated and subjected to a new vote. The level of evidence and degree of recommendation were classified according to the model proposed by the Center for Evidence-Based Medicine in Oxford.7
Preliminary ConsiderationsEvaluation of the Disease State in Systemic Lupus ErythematosusSLE activity fluctuates over time, and may be absent for varying periods and add accumulated damage or not in the course of the disease. Patients with SLE require standardized and objective monitoring with validated instruments to determine the degree of activity as precisely as possible and discriminate between active lesions and damage (LE 5, DR D, DA 100%). With the availability of new immunosuppressive drugs and BT for use in SLE it is particularly necessary for careful and expert monitoring of all aspects of the disease, using validated and reliable instruments.
Instruments to Measure Activity in Systemic Lupus ErythematosusTo evaluate disease activity there are several tools available. The simplest is the overall assessment of the activity by the physician (GPhA).8,9 However, this is subject to significant intra-and interobserver variability.10
Activity ratios were developed as objective tools for cohort studies of patients with SLE.11 They are known to be capable of predicting damage and mortality. In addition, they allow us to standardize the monitoring of SLE and assess more accurately the disease, as well as facilitate therapeutic decision making, though its usefulness in clinical practice is less well established. Like EULAR12 the panel recommends the use of a validated numerical index to assess overall SLE activity (LE 5, DR D, DA 100%). Although its use is not widespread in clinical practice, these indexes should be applied to all patients with SLE or at least those who are being treated with BT (LE 5, DR D, DA 93%).
Moreover, the panel recommended assessing disease activity in SLE every 15 days to 6 months depending on the prior clinical and laboratory data and changes in treatment (LE 5; DR D, DA 100%).
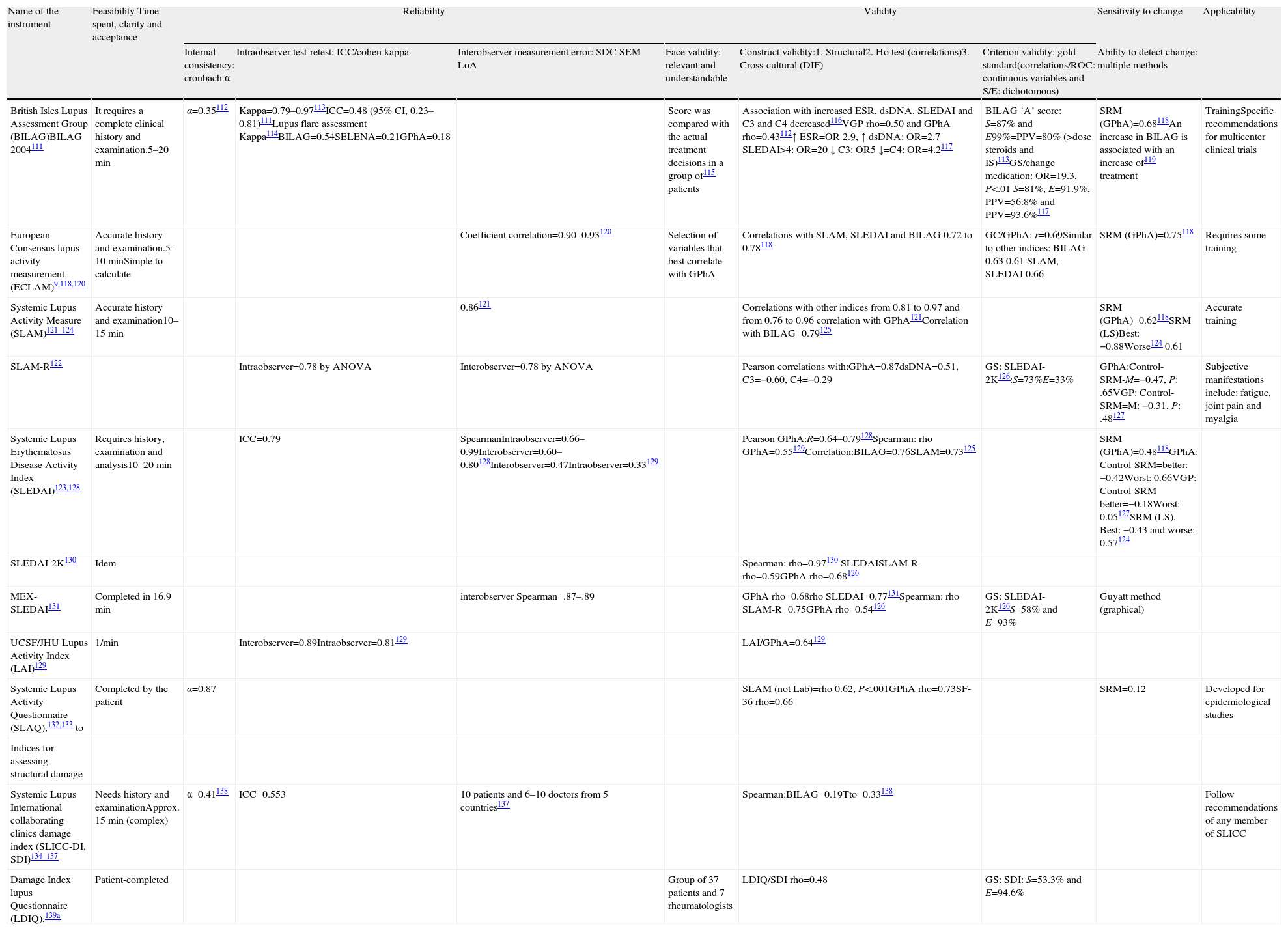
The characteristics of the instruments used are shown in Table 1. The panel recommends the use of SLEDAI preferably (and its updates: SELENA SLEDAI-2K or -SLEDAI) (LE 5, DR D, DA 93%). It is an overall validated numerical, brief, reliable, and simple tool to apply, even for non-experts.13,14 Other numerical global indices, such as ECLAM or SLAM-R, may be equally valid. The panel believes that, despite BILAG being the only activity index for organs or systems, the limited availability of the software tool (Blips) required for proper operation make it impractical to implement in daily practice. This index also requires specific training to ensure its proper implementation.
Indices of Activity and Damage in SLE.
| Name of the instrument | Feasibility Time spent, clarity and acceptance | Reliability | Validity | Sensitivity to change | Applicability | ||||
| Internal consistency: cronbach α | Intraobserver test-retest: ICC/cohen kappa | Interobserver measurement error: SDC SEM LoA | Face validity: relevant and understandable | Construct validity:1. Structural2. Ho test (correlations)3. Cross-cultural (DIF) | Criterion validity: gold standard(correlations/ROC: continuous variables and S/E: dichotomous) | Ability to detect change: multiple methods | |||
| British Isles Lupus Assessment Group (BILAG)BILAG 2004111 | It requires a complete clinical history and examination.5–20min | α=0.35112 | Kappa=0.79–0.97113ICC=0.48 (95% CI, 0.23–0.81)111Lupus flare assessment Kappa114BILAG=0.54SELENA=0.21GPhA=0.18 | Score was compared with the actual treatment decisions in a group of115 patients | Association with increased ESR, dsDNA, SLEDAI and C3 and C4 decreased116VGP rho=0.50 and GPhA rho=0.43112↑ ESR=OR 2.9, ↑ dsDNA: OR=2.7 SLEDAI>4: OR=20 ↓ C3: OR5 ↓=C4: OR=4.2117 | BILAG ‘A’ score: S=87% and E99%=PPV=80% (>dose steroids and IS)113GS/change medication: OR=19.3, P<.01 S=81%, E=91.9%, PPV=56.8% and PPV=93.6%117 | SRM (GPhA)=0.68118An increase in BILAG is associated with an increase of119 treatment | TrainingSpecific recommendations for multicenter clinical trials | |
| European Consensus lupus activity measurement (ECLAM)9,118,120 | Accurate history and examination.5–10minSimple to calculate | Coefficient correlation=0.90–0.93120 | Selection of variables that best correlate with GPhA | Correlations with SLAM, SLEDAI and BILAG 0.72 to 0.78118 | GC/GPhA: r=0.69Similar to other indices: BILAG 0.63 0.61 SLAM, SLEDAI 0.66 | SRM (GPhA)=0.75118 | Requires some training | ||
| Systemic Lupus Activity Measure (SLAM)121–124 | Accurate history and examination10–15min | 0.86121 | Correlations with other indices from 0.81 to 0.97 and from 0.76 to 0.96 correlation with GPhA121Correlation with BILAG=0.79125 | SRM (GPhA)=0.62118SRM (LS)Best: −0.88Worse124 0.61 | Accurate training | ||||
| SLAM-R122 | Intraobserver=0.78 by ANOVA | Interobserver=0.78 by ANOVA | Pearson correlations with:GPhA=0.87dsDNA=0.51, C3=−0.60, C4=−0.29 | GS: SLEDAI-2K126:S=73%E=33% | GPhA:Control-SRM-M=−0.47, P: .65VGP: Control-SRM=M: −0.31, P: .48127 | Subjective manifestations include: fatigue, joint pain and myalgia | |||
| Systemic Lupus Erythematosus Disease Activity Index (SLEDAI)123,128 | Requires history, examination and analysis10–20min | ICC=0.79 | SpearmanIntraobserver=0.66–0.99Interobserver=0.60–0.80128Interobserver=0.47Intraobserver=0.33129 | Pearson GPhA:R=0.64–0.79128Spearman: rho GPhA=0.55129Correlation:BILAG=0.76SLAM=0.73125 | SRM (GPhA)=0.48118GPhA: Control-SRM=better: −0.42Worst: 0.66VGP: Control-SRM better=−0.18Worst: 0.05127SRM (LS), Best: −0.43 and worse: 0.57124 | ||||
| SLEDAI-2K130 | Idem | Spearman: rho=0.97130 SLEDAISLAM-R rho=0.59GPhA rho=0.68126 | |||||||
| MEX-SLEDAI131 | Completed in 16.9min | interobserver Spearman=.87–.89 | GPhA rho=0.68rho SLEDAI=0.77131Spearman: rho SLAM-R=0.75GPhA rho=0.54126 | GS: SLEDAI-2K126S=58% and E=93% | Guyatt method (graphical) | ||||
| UCSF/JHU Lupus Activity Index (LAI)129 | 1/min | Interobserver=0.89Intraobserver=0.81129 | LAI/GPhA=0.64129 | ||||||
| Systemic Lupus Activity Questionnaire (SLAQ),132,133 to | Completed by the patient | α=0.87 | SLAM (not Lab)=rho 0.62, P<.001GPhA rho=0.73SF-36 rho=0.66 | SRM=0.12 | Developed for epidemiological studies | ||||
| Indices for assessing structural damage | |||||||||
| Systemic Lupus International collaborating clinics damage index (SLICC-DI, SDI)134–137 | Needs history and examinationApprox. 15min (complex) | α=0.41138 | ICC=0.553 | 10 patients and 6–10 doctors from 5 countries137 | Spearman:BILAG=0.19Tto=0.33138 | Follow recommendations of any member of SLICC | |||
| Damage Index lupus Questionnaire (LDIQ),139a | Patient-completed | Group of 37 patients and 7 rheumatologists | LDIQ/SDI rho=0.48 | GS: SDI: S=53.3% and E=94.6% | |||||
AUC: area under the curve E. specificity, GS: ‘gold standard’; LHR-: negative likelihood ratio; LHR+: positive likelihood ratio; LS: Likert scale; r: Pearson correlation coefficient; rho Spearman correlation coefficient; S: sensitivity; SRM standard response mean; GPhA: physician global assessment; PGA: patient's global assessment; NPV: negative predictive value; PPV: positive predictive value.
The panel recommended regular GPhA along with an overall numeric index, as it may provide additional information (LE 5, DR D, DA 79%).15
Definition of Clinical Activity and FlareClinical activity and flare are two relatable but not superimposable concepts. Conceptually, we define activity as a clinical setting activity in which the disease is not adequately controlled, resulting in different clinical and/or analytical manifestations. A flare refers to the reappearance of clinical activity at a particular time, in a previously controlled patient. Thus, activity is a more static concept, as it is something that persists more or less continuously over time, while a flare is more dynamic, as it is a change that occurs at a specific time. Although the activity concept is simple to understand, its categorization in an objective way is more complicated and has always been done arbitrarily. To this end, definitions based on previously described different activity indices have been used. The most used were SLEDAI (with different cutoffs) and BILAG (appearance of one BILAG A or two BILAG B), although others have proposed other definitions.
At present, we have no universally accepted definition of a flare or exacerbation of SLE.16,17 Recently, the Lupus Foundation of America has proposed the definition of a lupus flare as a measurable increase in activity in one or more organs with worsening or onset of new signs and symptoms and/or abnormalities in laboratory parameters.18 An alternative would be to use a definition of a disease flare linked to a cutpoint in an activity index, such as a change in SLEDAI ≥319 but this might lead to undetected flares because no representation is contained in an index or the flare is defined by serological change without clinical correlation. Finally, the SELENA study used a new version of the SLEDAI (SELENA-SLEDAI) and flares were defined and classified (into moderate and severe) using a new instrument, the SELENA-SLEDAI Flare Index (SFI), which used several cutting points for the activity index and also included the definitions of different types of clinical and therapeutic changes, as well as the physician global assessment.20 The panel proposed as the definition of a flare any potentially reversible clinical or biological changes contained in the GPhA activity indices or leading to an increase in treatment.
The panel recommends the systematic recording of lupus activity flares at each visit, preferably using an instrument like the SFI (SELENA-SLEDAI FLARE INDEX), although it has limited utility (LE 5, DR D, DA 86%).21
Measuring the Cumulative DamageAccumulated damage is defined as those clinically relevant irreversible lesions attributable to SLE, to the treatments employed or its associated complications.
It is recommended that we annually assess accumulated damage in all patients with SLE (LE 5, DR D, DA 100%). Currently there is only one available validated instrument, the SLICC/ACR/DI index.11,22
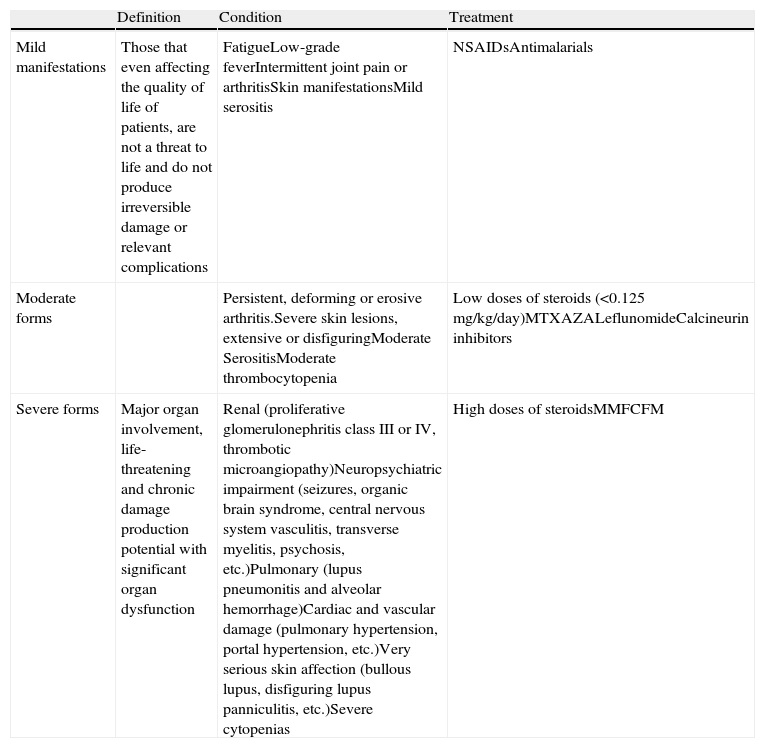
Classification of Systemic Lupus Erythematosus With Therapeutic PurposesSLE shows marked clinical heterogeneity in terms of the extent and severity. Consequently, the treatment of SLE should suit the patient's clinical status and disease severity. Generically, the clinical manifestations of SLE can be classified into 3 levels23,24: mild, moderate, and severe (Table 2).
Mild, Moderate and Severe Manifestations of SLE.
| Definition | Condition | Treatment | |
| Mild manifestations | Those that even affecting the quality of life of patients, are not a threat to life and do not produce irreversible damage or relevant complications | FatigueLow-grade feverIntermittent joint pain or arthritisSkin manifestationsMild serositis | NSAIDsAntimalarials |
| Moderate forms | Persistent, deforming or erosive arthritis.Severe skin lesions, extensive or disfiguringModerate SerositisModerate thrombocytopenia | Low doses of steroids (<0.125mg/kg/day)MTXAZALeflunomideCalcineurin inhibitors | |
| Severe forms | Major organ involvement, life-threatening and chronic damage production potential with significant organ dysfunction | Renal (proliferative glomerulonephritis class III or IV, thrombotic microangiopathy)Neuropsychiatric impairment (seizures, organic brain syndrome, central nervous system vasculitis, transverse myelitis, psychosis, etc.)Pulmonary (lupus pneumonitis and alveolar hemorrhage)Cardiac and vascular damage (pulmonary hypertension, portal hypertension, etc.)Very serious skin affection (bullous lupus, disfiguring lupus panniculitis, etc.)Severe cytopenias | High doses of steroidsMMFCFM |
NSAIDs: NSAIDs; AZA: azathioprine; CFM: cyclophosphamide; MMF: mycophenolate mofetil; MTX: methotrexate.
The goal of treatment of SLE is a complete clinical response, defined as the absence of perceived or demonstrable clinical activity and, ideally, the ability to discontinue immunosuppressive therapy in each case, as well as steroid treatment or at least reach a stable acceptable minimum dose (≤5mg/24h of prednisone or equivalent). Failing that, the therapeutic objective is tolerable minimum activity.25,26 The therapeutic goal should include the stability of disease, preventing new flares, new organ involvement and the development of irreversible tissue damage. No patient is considered subsidiary to treatment if serologically active in spite of being clinically quiescent.
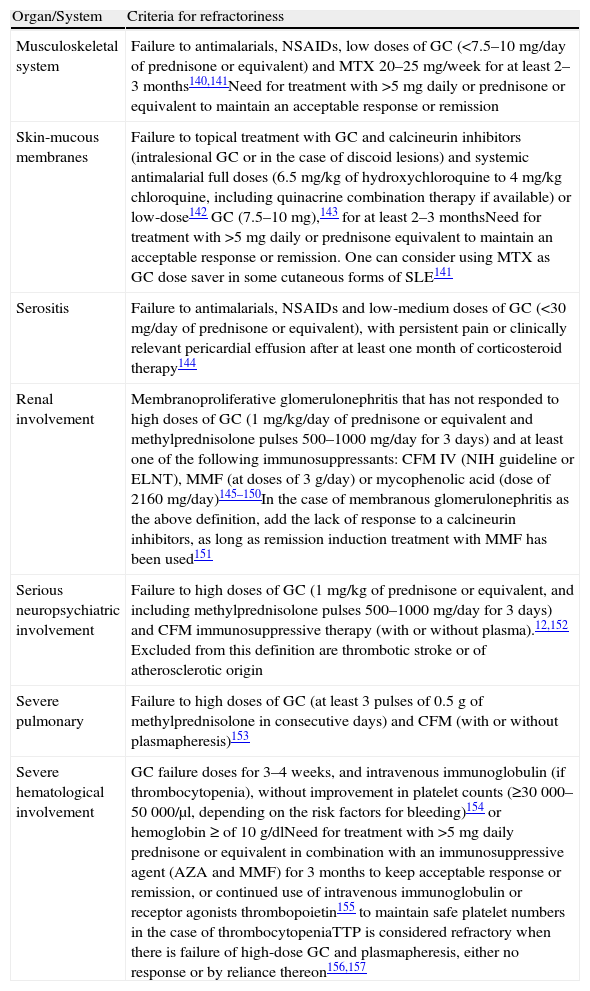
Refractory Systemic Lupus ErythematosusThere is no universally accepted definition of refractory SLE. This is understood as a patient who has not responded to standard treatment or requires an unacceptable glucocorticoid dose to maintain remission.27–31 One must always confirm that there has been compliance with the therapeutic regimen and consider cumulative damage, not likely to improve with treatment of SLE. The panel proposes the definition of refractory SLE for each organ or system that is detailed in Table 3.
Refractory SLE.
| Organ/System | Criteria for refractoriness |
| Musculoskeletal system | Failure to antimalarials, NSAIDs, low doses of GC (<7.5–10mg/day of prednisone or equivalent) and MTX 20–25mg/week for at least 2–3months140,141Need for treatment with >5mg daily or prednisone or equivalent to maintain an acceptable response or remission |
| Skin-mucous membranes | Failure to topical treatment with GC and calcineurin inhibitors (intralesional GC or in the case of discoid lesions) and systemic antimalarial full doses (6.5mg/kg of hydroxychloroquine to 4mg/kg chloroquine, including quinacrine combination therapy if available) or low-dose142 GC (7.5–10mg),143 for at least 2–3 monthsNeed for treatment with >5mg daily or prednisone equivalent to maintain an acceptable response or remission. One can consider using MTX as GC dose saver in some cutaneous forms of SLE141 |
| Serositis | Failure to antimalarials, NSAIDs and low-medium doses of GC (<30mg/day of prednisone or equivalent), with persistent pain or clinically relevant pericardial effusion after at least one month of corticosteroid therapy144 |
| Renal involvement | Membranoproliferative glomerulonephritis that has not responded to high doses of GC (1mg/kg/day of prednisone or equivalent and methylprednisolone pulses 500–1000mg/day for 3 days) and at least one of the following immunosuppressants: CFM IV (NIH guideline or ELNT), MMF (at doses of 3g/day) or mycophenolic acid (dose of 2160mg/day)145–150In the case of membranous glomerulonephritis as the above definition, add the lack of response to a calcineurin inhibitors, as long as remission induction treatment with MMF has been used151 |
| Serious neuropsychiatric involvement | Failure to high doses of GC (1mg/kg of prednisone or equivalent, and including methylprednisolone pulses 500–1000mg/day for 3 days) and CFM immunosuppressive therapy (with or without plasma).12,152 Excluded from this definition are thrombotic stroke or of atherosclerotic origin |
| Severe pulmonary | Failure to high doses of GC (at least 3 pulses of 0.5g of methylprednisolone in consecutive days) and CFM (with or without plasmapheresis)153 |
| Severe hematological involvement | GC failure doses for 3–4 weeks, and intravenous immunoglobulin (if thrombocytopenia), without improvement in platelet counts (≥30000–50000/μl, depending on the risk factors for bleeding)154 or hemoglobin ≥ of 10g/dlNeed for treatment with >5mg daily prednisone or equivalent in combination with an immunosuppressive agent (AZA and MMF) for 3 months to keep acceptable response or remission, or continued use of intravenous immunoglobulin or receptor agonists thrombopoietin155 to maintain safe platelet numbers in the case of thrombocytopeniaTTP is considered refractory when there is failure of high-dose GC and plasmapheresis, either no response or by reliance thereon156,157 |
NSAIDs: NSAIDs; AZA, azathioprine; CFM: cyclophosphamide; ELNT: Eurolupus Nephritis Trial; GC: glucocorticoids; SLE: systemic lupus erythematosus; MTX: methotrexate; MMF: mycophenolate mofetil; NIH: National Institute of Health; TTP thrombocytopenic purpura thrombocytopenic.
Currently, BLM is the only approved BT with an indication in SLE, although rituximab, tocilizumab, abatacept (ABT) and TNF-α inhibitors have been used off-label.
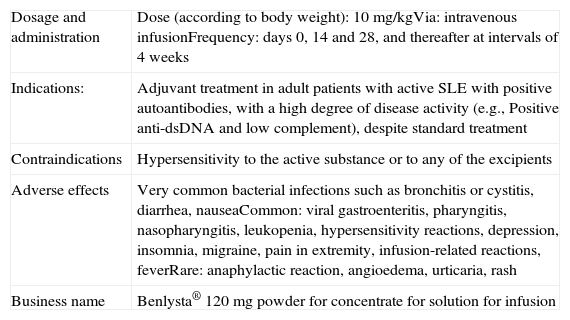
Table 4 summarizes the BLM data sheet. The technical summary of biological treatments without approved indications in SLE can be found in the SER Consensus document on risk management of treatment with biological therapies in patients with rheumatic diseases.32
Summary Information Sheet for Belimumab.
| Dosage and administration | Dose (according to body weight): 10mg/kgVia: intravenous infusionFrequency: days 0, 14 and 28, and thereafter at intervals of 4 weeks |
| Indications: | Adjuvant treatment in adult patients with active SLE with positive autoantibodies, with a high degree of disease activity (e.g., Positive anti-dsDNA and low complement), despite standard treatment |
| Contraindications | Hypersensitivity to the active substance or to any of the excipients |
| Adverse effects | Very common bacterial infections such as bronchitis or cystitis, diarrhea, nauseaCommon: viral gastroenteritis, pharyngitis, nasopharyngitis, leukopenia, hypersensitivity reactions, depression, insomnia, migraine, pain in extremity, infusion-related reactions, feverRare: anaphylactic reaction, angioedema, urticaria, rash |
| Business name | Benlysta® 120mg powder for concentrate for solution for infusion |
BLM is a human monoclonal antibody that inhibits soluble B lymphocyte stimulator, a key cytokine to the survival of B cells, which is overexpressed in33–39 SLE.
Basic StudiesIn the initial phase II placebo-controlled trial, BLM did not achieve the objectives of efficiency based on the SELENA-SLEDAI activity index at weeks 24 and 52.40 However, post hoc analysis showed that BLM significantly reduced disease activity in patients with anti-nuclear (ANA) and anti-DNA positive antibodies. This is why the BLM registration trials (BLISS-5241 and BLISS-7642 included only patients with positive ANA and/or anti-dsDNA, with SELENA-SLEDAI of ≥6. Patients were randomized to receive intravenous infusions 1mg/kg of BLM, 10mg/kg of BLM or placebo on days 0, 14, and 28 and then every 28 days. The primary outcome measure was a response on the SLE Reply Index (SRI) at week 52, defined as a ≥4-point reduction in the SELENA-SLEDAI, no BILAG A, no more than one BILAG B or lack of worsening in GPhA.
In BLISS-5241 there were significantly higher percentages of patients with a response and an SRI reduction ≥4 points in the SELENA-SLEDAI, and lower rates of new BILAG A or B and GPhA worsening in the BLM vs the placebo group. In BLISS-7642 a higher percentage of patients in the BLM 10mg/kg group achieved an SRI response at week 52 compared to placebo (P=.017). In two randomized clinical trials (RCTs), the rate of adverse events was similar across treatment groups.
Use in Specific SituationsThere is no evidence in connection with the use of BLM in manifestations of SLE such as lupus nephritis or severe neurological manifestations, as patients with these two types of involvement were specifically excluded from the trials. In a post hoc analysis of the fundamental phase III trials, a beneficial effect of BLM on renal outcome measures in patients with non-severe renal impairment has been suggested.43 Likewise, another recently published post hoc analysis has shown a beneficial effect on cutaneous and musculoskeletal manifestations.44,45
RecommendationThe panel recommends the use of BLM in adult patients with active SLE with positive autoantibodies and high disease activity despite standard therapy (LE 1; DR, DA 93%). Today, patients with refractory non-major clinical manifestations (such as arthritis or skin involvement) and laboratory activity seem to be the most appropriate clinical setting for use of this agent.
Currently, we cannot recommend the use of BLM in patients with SLE and severe involvement of the central nervous system (CNS) and/or severe lupus nephritis (LE 1; DR, DA 93%).
Pharmacovigilance and Risk ManagementAlthough the safety profile shown by BLM in clinical trials was good, there is still little experience in long term use. Follow-up experience of the first patients entered into clinical trials with this drug and who continued using it in later extension stages for 4 years that was recently published (total experience of 1165 patient-years), observed that the incidence of adverse effects, including infusion reactions, infections, malignancies, laboratory abnormalities, or the number of withdrawals from treatment due to unwanted effects, were stable or declining.46
In a patient with SLE who is to initiate treatment with BLM, it is important to consider the possible co-existence of infections, cancer, heart failure, cytopenias or other significant comorbidity that should be monitored, or a contraindication to starting treatment.
An active systemic or localized infection is a contraindication for the start of BLM (LE 2b, DR B, DA 100%). In case of chronic, recurrent or high risk of infection, risk-benefit of its employment should be assessed strictly for close monitoring. One must also pay special attention to the possible development of infections during treatment. If this occurs, diagnosis and treatment of cases are essential and the temporary suppression of BLM. Once infection is resolved, you can restart BT.
Risk of BLM use in patients with active or latent tuberculosis (BT) is unknown. The panel advises to follow the general recommendations for the use of biological therapies in rheumatic diseases, which can be found on the BE Consensus document on managing the risk of treatment with biological therapies in patients with rheumatic diseases.32
Prior to the start of the BLM, one must take into account the history of malignancies and lymphoproliferative disorders (LE 5, DR D, DA 100%). When a history of this exists, special caution is needed for its use, although no evidence of increased risk of solid tumors in patients treated with BLM. If malignancy is diagnosed during treatment with BLM, it should be suspended.
Before starting treatment with BLM, the existence of cytopenias must be evaluated. If attributed to SLE, treatment could be started cautiously. Otherwise, one should not start treatment until resolution (LE 5, DR D, DA 100%). If the patient develops severe cytopenia during treatment with BLM, we recommend stopping and looking for other potential causes. After resolution, treatment may be restarted.
HBV and HCV serology is advised. Administration of pneumococcal and influenza vaccine prior to starting treatment with BLM is recommended (LE 3b, DR C, DA 100%). We discourage the use of vaccines with live or attenuated germs within 30 days or simultaneously with the use of BLM.
BLM is not recommended in patients >65 years, unless the expected benefits outweigh the risks. It is also not recommended for use in patients with CNS manifestations, severe active lupus nephritis, human immunodeficiency virus infection, a history of or active HBV or HCV infection, hypogammaglobulinemia (IgG <400mg/dl) or IgA deficiency (IgA <10mg/dl), a history of major organ transplant or hematopoietic stem cell/bone marrow transplantation or kidney transplant, as it has not been used in those situations (LE 5, DR D, DA 93%).
BLM is not recommended for use with other treatments acting on B cells or cyclophosphamide (CFM) (LE 5, DR D, DA 93%).
BLM does not require dose adjustment in patients with mild, moderate or severe renal failure. However, due to the absence of data, caution is recommended in patients with severe renal impairment (LE 5, DR D, DA 93%).
Pregnancy should be avoided during treatment with BLM and in the 4 months after completion. BLM should not be used during pregnancy unless clearly necessary (LE 5, DR D, DA 100%). Regarding breastfeeding, you should consider the benefit of it to the child and the benefit of treatment with BLM for mother and decide.
In patients who are to undergo a surgery in the absence of sufficient experience to date, the panel recommends BLM suspended in the 2 weeks before surgery and resumed 2–4 weeks after surgery, given the increased risk of infections, cytopenias, and coagulation disorders in this period (LE 5, DR D, DA 100%).
During follow-up, conduct a clinical and laboratory evaluation of the patient, with a periodicity of monthly visits at baseline and then 1–3 months, depending on the patient's progress (LE 5, DR D, DA 100%).
Biologic Therapy Without an Authorized Indication in Systemic Lupus ErythematosusRituximabProduct DescriptionRituximab (RTX) is a soluble chimeric monoclonal antibody directed against the CD20 membrane receptor, present in most cellular phases of B cell ontogeny (from pre-B cell to memory B cell), but not in the plasma cell. Infusing this antibody produced, by various mechanisms (cytotoxicity mediated and non-mediated by antibodies and antibody-mediated apoptosis) a depletion of the population of cells over a period of 6 months or more.
Major StudiesCurrently, RTX has no approved indication for SLE. However, there are numerous open studies that support the use of RTX in various situations, including some serious such as nephritis, cytopenias or nervous system involvement. However, the EXPLORER study was unable to prove superiority of RTX vs placebo associated with conventional treatment in patients with active lupus in its primary stage (whose objective was to achieve and maintain a clinical response at week 52 using an 8 organ BILAG).47 This double-blind Phase II/III RCT evaluated the efficacy and safety of RTX (2 doses of 1g separated by 14 days) or placebo added to standard therapy (varying doses of glucocorticoids, azathioprine [AZA], mycophenolate mofetil [MMF] or methotrexate [MTX]) in 257 patients with lupus without renal involvement or with moderate to severe activity. They have suggested various design issues that could explain this unfavorable outcome such as it being underpowered to demonstrate differences between 2 groups of patients with active medication, the use of varying doses of glucocorticoids and immunosuppressant, limited follow up time (one year may not allow a deferred evaluation of therapeutic effects) or insensitivity to the improvement criteria. Another RCT conducted in patients with lupus nephritis, the LUNAR study,48 which will be discussed below, also identified an additional benefit associated to the use of RTX. However, this study also suffered from methodological problems similar to the EXPLORER.49
The specific dose of RTX for SLE has not been established. In clinical trials it has generally been used in the same way as in rheumatoid arthritis, infusing two 1g doses47,48,50–52 14 days apart. However, numerous studies have used it as in lymphoma treatment, 4 weekly infusions of 375mg/m.2,53,54 It has even been suggested that a pattern shorter than 4 weekly infusions of 100mg may be effective.55
The convenience of managing RTX as monotherapy or in combination with other immunosuppressants has not been established. In most of the open studies 2 intravenous doses of 500–750CFMmg on days 3 and 17 have been associated to the standard dose of 375mg/m2 of RTX. However, this combination has not demonstrated any advantage in the induction treatment of lupus nephritis.56 in the LUNAR48 study, all patients received MMF concomitantly, which does not allow the drawing of conclusions on the desirability of the combination of drugs. Recently, the appearance of progressive multifocal leukoencephalopathy has been seen in SLE patients treated with RTX with previous or concomitant use of alkylating agents.57
In the AIR58 registry, the combination with any immunosuppressant, including CFM, obtained significant drops in SELENA-SLEDAI scores 6±3 months after infusion and longer times to recurrence.59 In the LESIMAB cohort, any concomitant immunosuppressant other than the CFM were 3.5 times more likely to achieve a clinical response after a mean follow-up of 20±15.2 months. In the series by Vital et al.,60 patients who were receiving concomitant immunosuppressive outperformed monotherapy patients, with no differences between the different drugs.
The panel recommends keeping previous immunosuppressive therapy in patients with SLE in which treatment was started if there was an inadequate response to them (LE 4, DR C, DA 86%). However, the panel calls attention to the potential risks of combined use with certain immunosuppressants, such as CFM, in which case the risk should be balanced against the seriousness of the event in question (LE 5; DR D, DA 93%).
Patients in particularly serious situations have also been used in combination with bolus glucocorticoids and RTX or without CFM bolus. Glucocorticoid doses also varied, depending on the patient.
In the LUNAR and EXPLORER RCTs, the RTX dose was repeated at 6 months after initial treatment and the observational study by Turner-Stokes et al.61 suggests that the constant repetition of doses of RTX is more convenient than on-demand treatment. However, and even though it seems that the majority of SLE patients require repeated cycles, it has been more widely used on demand in patients with an initial response. In the absence of lupus activity, the panel does not recommend the routine administration of new cycles of RTX, except in patients with particularly severe flares of disease, in which a new flare could bring dire consequences (LE 5, DR D, DA 79%).
Use in Specific SituationsLupus NephritisThe LUNAR study48,50,51 compared the efficacy of MMF in lupus nephritis and glucocorticoids vs its association with RTX. Although RTX failed to improve clinical outcome measures, there was a greater reduction in the levels of anti-dsDNA and complement with respect to the control patients. Uncontrolled open studies in patients refractory to other immunosuppressives have shown efficacy of RTX in lupus nephritis.58,62 In the AIR registry,58 of the 42 patients with nephritis refractory to conventional therapy who received RTX, 48% achieved a complete response and 23% a partial response. In the UK BIOGEAS62 series of 164 patients in the refractory nephritis group treated with RTX, 67% achieved response, proteinuria improved in 90% of patients and improved glomerular filtration rate in half of the cohort. In the LESIMAB59 cohort, 82% of the 63 patients achieved a renal response after treatment with RTX. Finally, a systematic review published in 201063 found that 29% of nephritis patients treated with RTX achieved a complete response and 37% achieved a partial response.
Therefore, despite not having demonstrated a clear positive effect added to treatment with MMF in clinical trials, RTX may be an effective drug in the management of patients refractory to standard immunosuppressives, especially CFM and MMF (LE 2b, DR B, DA 93%).
ArthritisThere are no clinical trials that specifically evaluate the efficacy and safety of RTX in SLE arthritis. In the cohort LESIMAB, 93% of patients with active arthritis undergoing treatment responded to RTX.59 A review of 100 patients also observed this RTX efficiency at the articular level.64 Of the 36 patients with arthritis treated with RTX in the AIR registry on record, 52% achieved complete remission and 20% partial remission after treatment with RTX.58 Finally, in the retrospective series of 52 patients published by Garcia-Carrasco et al.,65 76% of the 25 who had severe arthritis achieved complete remission after 6 months of treatment. Based on data from these open studies, it is considered that RTX is effective in treating arthritis in SLE (LE 4; DR C; DA 93%).
Central Nervous System InvolvementExperience with RTX in neurolupus is scarce and limited to several case series. In a series of 22 patients with CNS lupus treated with RTX,66 72% experienced rapid improvement, while in the remaining 28% the disease progressed or remained stable. The type of damage that most improved was acute confusional state, followed by cognitive dysfunction, psychosis and seizures.67 In another series of 12 neurolupus patients treated with RTX, improvement was observed in 9 (4 with myelopathy, 4 with polyneuropathy and one with polyneuropathy and seizures). In the LESIMAB59 cohort, 11 patients received RTX for neurolupus, showing favorable results in over 70% of cases. Therefore, RTX can be used in patients with diffuse forms of neurolupus, cognitive deficits, psychosis or seizures (LE 4; DR C, DA 93%).
Hematologic InvolvementThere have been numerous case series of patients with cytopenias, mainly thrombocytopenia treated with RTX. In a retrospective series68 of 31 patients with SLE and steroid-refractory and immunoglobulin thrombocytopenia, 56% of patients normalized platelet counts after 6 months of treatment with RTX. Other series with less than 10 patients have obtained similar results.54,64,65,67 In the series of 10 patients by Chen et al.,55 70% achieved a complete response (>50000 platelets) after receiving four infusions of only 100mg of RTX. There is also a case with good response to RTX after failing splenectomy.69
RTX is a valid treatment option in thrombocytopenia or other cytopenias refractory to glucocorticoids, hydroxychloroquine and at least one immunosuppressant (AZA or MMF) (LE 4, DR C, DA 93%).
Recommendation of useRTX should be reserved for patients with a moderately or severely inadequate response, or resistant to standard treatment with corticosteroids, immunosuppressants (AZA, MTX, MMF, CFM or calcineurin inhibitors) and BLM (depending on the event in question) (LE 2b, DR B, DA 93%). The need for a relatively high dose of glucocorticoids to maintain control of the disease may also be an indication (Table 5).
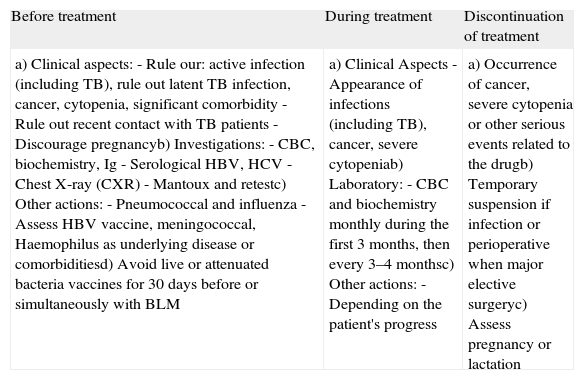
Assessment Prior to Prescribing a Biologic Drug and Control During Treatment in Patients With SLE.
| Before treatment | During treatment | Discontinuation of treatment |
| a) Clinical aspects:- Rule our: active infection (including TB), rule out latent TB infection, cancer, cytopenia, significant comorbidity- Rule out recent contact with TB patients- Discourage pregnancyb) Investigations:- CBC, biochemistry, Ig- Serological HBV, HCV- Chest X-ray (CXR)- Mantoux and retestc) Other actions:- Pneumococcal and influenza- Assess HBV vaccine, meningococcal, Haemophilus as underlying disease or comorbiditiesd) Avoid live or attenuated bacteria vaccines for 30 days before or simultaneously with BLM | a) Clinical Aspects- Appearance of infections (including TB), cancer, severe cytopeniab) Laboratory:- CBC and biochemistry monthly during the first 3 months, then every 3–4 monthsc) Other actions:- Depending on the patient's progress | a) Occurrence of cancer, severe cytopenia or other serious events related to the drugb) Temporary suspension if infection or perioperative when major elective surgeryc) Assess pregnancy or lactation |
BLM: belimumab; Rx: radiography; TB: tuberculosis; HBV: hepatitis B virus; HCV: hepatitis C.
In relation to risk management, retreatment with RTX is safe for at least 6–12 months.70 In the absence of specific pharmacovigilance data, we recommend following the risk management directions issued for RTX use in other chronic inflammatory joint diseases (LE 5, DR D, DA 93%).
Tumor Necrosis Factor AntagonistsProduct DescriptionLevels of serum TNF-α are greatly increased in SLE patients and correlate with disease activity.71–73 That is why, a priori, their blockade could be effective in treating these patients.
Major StudiesIn relation to the available evidence on efficacy and safety with these agents in SLE, it should be noted that only 2 RCTs with infliximab (IFX) and etanercept (ETN) double blind placebo prematurely closed to,74,75 for what is currently available data are based on small uncontrolled open studies or case series.
In a publication showing data76 of 13 patients refractory to standard therapy, 6 from a previous open study77 and 7 from various centers, it was found that a cycle of four IFX infusions improved immunosuppression with lupus nephritis (III, IV, V) in terms of improvement of >50% in proteinuria, and stabilization of serum creatinine in 7 of 9 patients, 4 of which remained stable over time (up to 5 years) and 3 worsened despite new IFX cycles. The 5 patients with arthritis went into remission, but chronic treatment maintained its effect only in 2 patients. In another open study with 11 patients78 it was found that IFX treatment for 6 months was superior to standard therapy in the improvement in SLEDAI but no difference was seen in steroid dose changes, SF-36, SLICC/ACR/DI, proteinuria, complement or autoantibody levels. A third open IFX study showed very similar results to the prior ones.79 On the other hand, there are cases or case series on the use of IFX in patients with pulmonary involvement or hemphagocytic lymphohistiocytosis.76,80–82
There are some case reports of patients with SLE and ETN.83–85 Similarly, there is insufficient evidence regarding the use of adalimumab, certolizumab and golimumab, or on the use of anti-TNF-α for other relevant clinical manifestations of SLE.
Use in Special Situations. RecommendationsIn patients with corticosteroid refractory lupus nephritis and immunosuppressants, including CFM, AZA and MMF, and RTX, the possibility of a short course of anti-TNF and off-label therapy can be assessed (LE 4, DR C, DA 73%).
In patients with SLE and arthritis, using anti-TNF agents could be justified only in cases of failure to standard immunosuppressive therapy, as well as other biological therapies (or inability to use them), such as BLM, RTX and probably ABT. In any case, its use should be only after a careful analysis of the risk/benefit (LE 5, DR D, DA 73%).
Pharmacovigilance and Risk ManagementRegarding risk management of the use of anti-TNF, given the small number of patients and the exposure time we cannot draw conclusive data. There are reports of serious infections and lymphoma. It has also been observed that short term treatment with IFX leads to transient increases of autoantibodies in the long term and may increase the levels of anti-dsDNA.76 In the same way, there is at least one case described of a patient who developed deep vein thrombosis that could be related to the medication, suggesting that it may be more common in type V lupus nephritis.86
In the absence of specific pharmacovigilance data, we recommend following the directions issued on risk management on the use of anti-TNF in other chronic inflammatory joint diseases (LE 5, DR D, DA 100%).
Caution is advised in patients with nephrotic syndrome and/or massive proteinuria (more common in type V lupus nephritis), in whom anti-TNF used should be balanced against the potential risk of developing deep vein thrombosis (LE 5, DR D, DA 86%).
AbataceptProduct DescriptionABT is a fusion protein consisting of the extracellular domain of human CTLA-4 linked to an Fc fragment of human IgG1. ABT selectively inhibits lymphocyte costimulation blocking the binding of CD80/CD86 to CD28 without causing T cell depletion.87
Major StudiesThe efficacy and safety of ABT in patients with SLE have been studied in RCTs in two distinct situations, on the one hand, in patients with active extrarenal manifestations88 and, more recently, in patients with lupus nephritis.89
A double-blind, placebo controlled trial88 evaluated the efficacy and safety of ABT in SLE patients without renal involvement. It enrolled 180 patients with active disease (polyarthritis, serositis and discoid lupus), who were randomized to receive ABT 10mg/kg or placebo for one year. All patients received prednisone (30mg/day for a month and then tapering). No significant differences in the proportion of patients with a new flare of SLE (BILAG A or B) after starting prednisone tapering or any of the secondary BILAG dependent objectives were seen.
A post hoc analysis showed a greater efficacy of ABT in polyarthritis (62.5% vs 36.5%), assessment of the flare by the physician (82.3% vs 63.6%) and the proportion of patients without recurrence during months 10–12 when treated with doses of 7.5mg/day of prednisone (42.4 vs 28.1) or lower. ABT showed some efficacy in patient-dependent variables (SF-36, fatigue or sleep disorders). The frequency of serious adverse events was higher in the ABT group (19.8 vs 6.8).
Another double-blind, placebo-controlled study89 evaluated the efficacy and safety of ABT in 298 patients with active class III or IV lupus nephritis. Patients received ABT 10mg/kg/month for 12 months, or 30mg/kg/month for 3 months followed by 10mg/kg/m or placebo for 9 months to one year. All patients received MMF 1.5–3g/d, prednisone (up to 60mg/day for a month, and then in descending pattern). No significant differences between groups in terms of time to complete renal response or in the secondary objectives were seen. In a subanalysis of patients with nephrotic syndrome (n=122), there was a trend toward greater ABT efficacy in reducing proteinuria after the sixth month, maintained until the end of the study.
These negative results contrast with ABT in RCT data from animal models, and clinical experience outside of clinical trials. Study design aspects, such as its short duration, non-validated outcomes, heterogeneous populations or concomitant use of highly effective therapies appeared to contribute clearly to the negative results of these RCTs.90,91 In the case of ABT, the possible efficacy in subgroups of patients with polyarthritis or nephrotic syndrome suggests at least some usefulness in some patients with SLE.92
Recommendation of useThe panel does not recommend the use of ABT for the treatment of SLE, or its renal and extrarenal manifestations (LE 1; DR, DA 93%).
However, in patients with polyarthritis refractory to other therapies, and in which no other therapeutic option was possible, ABT may be useful (LE 4; DR D; DA 86%).
Pharmacovigilance and Risk ManagementIn the absence of specific pharmacovigilance data, we recommend following the directions issued on ABT risk management used for other chronic inflammatory joint diseases (LE 5, DR D, DA 100%).
TocilizumabProduct DescriptionTocilizumab (TCZ) is a humanized monoclonal antibody directed against the receptor for IL-6. It suppresses both soluble and membrane-bound IL-6 receptor-mediated signaling soluble IL-6.93 In SLE there is increased serum IL-671,73,86,94–96 which is clinically related to activity and the concentration of anti-dsDNA antibodies.
Major StudiesThere are no double-blind RCTs demonstrating the efficacy of TCZ in the treatment of SLE. Shirota et al.97 reported in 2005 the results of a study in which TCZ infusions were administered biweekly at doses of 2mg/kg (n=4), 4mg/kg (n=6) or 8mg/kg (n=3) for 12 weeks to patients with SLE, with subsequent follow-up visits at 8 weeks. It was observed that treatment with TCZ decreases lymphocyte activation, the homeostasis of T and B cells, blocking their differentiation and/or trafficking in SLE patients, normalizing abnormal T cell and B subsets.
In an open dose escalation98 study which evaluated the safety and efficacy of TCZ in 16 patients with moderately active SLE, 7 infusions were administered biweekly (2mg/kg in 4 patients, 4mg/kg in 6 patients and 8mg/kg in another 6) for 12 weeks, with a follow-up of 8 weeks. There was dose-related neutropenia, recovering after discontinuation of treatment. Unrelated infections were observed in 11 cases. There was an improvement in the SELENA-SLEDAI score in 8 of 15 patients and improvement in 7 patients that had arthritis at baseline and which resolved completely in 4. However, no improvement of proteinuria was seen in the 5 patients with nephropathy. The levels of anti-dsDNA IgG and decreased in the 4 and 8mg/kg groups, suggesting a specific of TCZ on autoantibody producing cells. Although TCZ has been associated to increased ALT/AST and cholesterol related to RA,99,100 the latter study98 showed no significant changes in the serum concentrations in patients with SLE. In all cases there was a significant decrease in erythrocyte sedimentation rates and C reactive protein levels.
Recommendations for UseThe panel proposes to consider the use of TCZ in selected patients with SLE refractory to conventional treatments (hydroxychloroquine, corticosteroids, AZA, MTX, cyclosporine, and MMF CFM) who do not respond or are intolerant to BLM and RTX (LE 4, DR C, DA 86%).
Pharmacovigilance and Risk ManagementRegarding risk management of the use of TCZ, given the low patient volume and exposure time, no conclusive data can be extracted. Neutropenia has been described and it has been observed that short term treatment with transient increases transaminases, triglycerides and cholesterol occurs in patients with RA treated with TCZ, although this effect was not observed in SLE.
In the absence of specific pharmacovigilance data, we recommend following the TCZ use directions issued on risk management in other chronic inflammatory joint diseases (LE 5, DR D, DA 100%).
Experimental TherapiesIntroductionOther treatments currently in development include drugs specific for B-cell costimulatory molecules, cytokines, and specific intracellular signaling pathways. These include epratuzumab, atacicept, sifalimumab rontalizumab and monoclonal antibodies directed against specific B cell surface antigens CD22, TACI and interferon-alpha (IFN-α) (the last 2 agents), respectively.
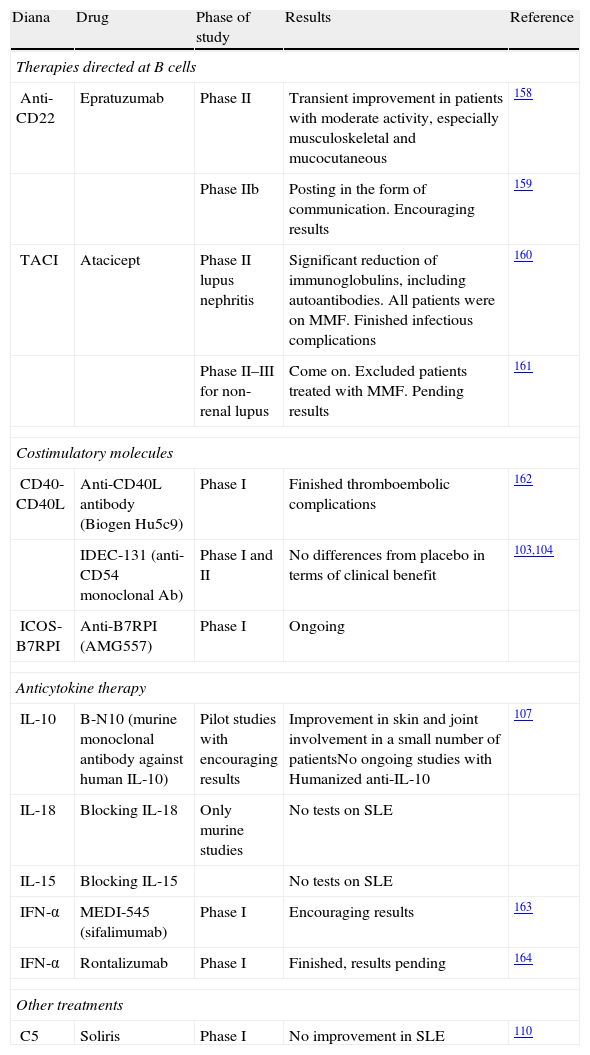
Below we give a brief explanation of the mechanism of action of each molecule. Available studies are summarized in Table 6.
Experimental Therapies.
| Diana | Drug | Phase of study | Results | Reference |
| Therapies directed at B cells | ||||
| Anti-CD22 | Epratuzumab | Phase II | Transient improvement in patients with moderate activity, especially musculoskeletal and mucocutaneous | 158 |
| Phase IIb | Posting in the form of communication. Encouraging results | 159 | ||
| TACI | Atacicept | Phase II lupus nephritis | Significant reduction of immunoglobulins, including autoantibodies. All patients were on MMF. Finished infectious complications | 160 |
| Phase II–III for non-renal lupus | Come on. Excluded patients treated with MMF. Pending results | 161 | ||
| Costimulatory molecules | ||||
| CD40-CD40L | Anti-CD40L antibody (Biogen Hu5c9) | Phase I | Finished thromboembolic complications | 162 |
| IDEC-131 (anti-CD54 monoclonal Ab) | Phase I and II | No differences from placebo in terms of clinical benefit | 103,104 | |
| ICOS-B7RPI | Anti-B7RPI (AMG557) | Phase I | Ongoing | |
| Anticytokine therapy | ||||
| IL-10 | B-N10 (murine monoclonal antibody against human IL-10) | Pilot studies with encouraging results | Improvement in skin and joint involvement in a small number of patientsNo ongoing studies with Humanized anti-IL-10 | 107 |
| IL-18 | Blocking IL-18 | Only murine studies | No tests on SLE | |
| IL-15 | Blocking IL-15 | No tests on SLE | ||
| IFN-α | MEDI-545 (sifalimumab) | Phase I | Encouraging results | 163 |
| IFN-α | Rontalizumab | Phase I | Finished, results pending | 164 |
| Other treatments | ||||
| C5 | Soliris | Phase I | No improvement in SLE | 110 |
ICOS: inducible costimulator; IFN-α, interferon-alpha; IL: interleukin; SLE: systemic lupus erythematosus; MMF: mycophenolate mofetil; TACI: transmembrane activator and calcium modulator and cyclophilin ligand interactor.
The CD22 antigen is a 135kDa glycoprotein that is expressed in and restricted to B lymphocytes and participates regulating its activation and interaction with T cells.101 It is expressed in the cytoplasm of pro-B and pre-B cells, and on the surface of mature B cells. Epratuzumab is a recombinant anti-CD22monoclonal antibody, with a 90%–95% human origin. It primarily has an immunomodulatory effect on B cells, unlike RTX, which is primarily a cytotoxic agent.
AtaciceptAtacicept is a chimeric fusion protein of the extracellular domain of Transmembrane Activator and Calcium receptor modulator and cyclophilin ligand Interactor (TACI) domain linked to human IgG1, which blocks the stimulation of B cells dependent on Blys and APRIL. Studies in mouse models and in humans have shown a marked reduction in the levels of immunoglobulins (including autoantibodies) after treatment, due to its effect on plasma cells.
Blocking Regulatory SignalsCostimulation signals promote T-cell differentiation, proliferation and activation of B cells Of all known costimulatory pathways, in SLE those studied have only been those specifically composed by CD40-CD40 ligand (CD40L or CD154) and B7-CD28. CD40L-neutralizing antibody may interfere with reactions occurring in the germinal centers and also reduce activation of B lymphocytes in the marginal zone.102 Some studies in SLE patients have been prematurely ended due to thromboembolic complications.
There have been other three trials with IDEC-131, a monoclonal antibody against CD154, which have included a total 110 patients103–105 and have demonstrated good safety and tolerability in patients with SLE. Regarding efficiency, no significant differences over placebo have been achieved.105
The inducible costimulator (ICOS) is a T cell-specific molecule, structurally and functionally related to CD28. It is induced on the surface of T cells after these are activated. It transmits costimulation signals in T cell. ICOS and its ligand are involved in the interaction between T and B cells, and differentiation of the latter. Although it looks like a promising therapeutic target in murine lupus models, there is no experience in humans.106 Finally, and related to costimulatory molecules, there is now a phase I trial with anti-B7RP1 (AMG557) underway in patients with SLE.
Anticytokine TreatmentsAlthough the role of IL-10 in the pathogenesis of SLE is not completely defined, it seems to be involved in B cell hyperactivation and antibody production. A murine monoclonal antibody has been developed which neutralizes human IL-10 (B-N10), with a small open study in 6 patients showing improvement in clinical activity and reduced use of prednisone. However, this agent has not had any further clinical development.107
IFN-a is a pleiotropic cytokine that is essential in the pathogenesis of SLE.108 Its levels are increased in serum of patients with SLE, the gene expression profile of the peripheral blood mononuclear cells is dependent on IFN-α and, finally, the serum of SLE patients is able to induce the maturation of dendritic cells, which is also dependent effect of IFN-α. Almost two-thirds of patients with SLE show common features related to IFN-α. In mice, its involvement has been demonstrated in B cell dependent lymphopenia, differentiation of germinal centers and generation of plasma cells. Hence it is considered as a very good therapeutic target. To date, two monoclonal antibodies against IFN-α have been tested, sifalimumab and rontalizumab. However, results of a phase II of the latter agent109 recently reported seem to show no differences from placebo.
Other TargetsRecently, new, humanized anti-CD20 monoclonal antibodies have been designed, such as ofatumumab (HuMax CD20), IMMU-106 (hA20), GA-101, with high cytotoxic potential on B cells. Experience is limited to preclinical studies and they have not been tested yet in patients with SLE.
Finally, eculizumab is an antibody that specifically inhibits terminal activation of complement.110
ConclusionsAs in other rheumatic diseases, biological drug treatments have burst onto the SLE scene. In fact, the first drug that has been approved in the last 5 decades as a specific treatment for SLE has been a monoclonal antibody that blocks the action of a cytokine essential for the survival and maturation of B lymphocytes. However, SLE, unlike other types of diseases, is an especially heterogeneous entity, with different clinical manifestations, and therefore poses enormous difficulties in performing standardized and objective assessments of its clinical situation in a given moment in time, as well as its responses to different therapeutic efforts. All this has made it difficult, in many cases, to develop trials or have led to their results to being inconclusive.
Due to the above, the approval of BLM as specific therapy for SLE has been a significant event in this disease and may mark a turning point in its treatment. However, the clinical indication specified in the data sheet of the product is somewhat non-specific and, therefore, hinders a clear positioning on this agent in the therapeutic arsenal against SLE.
This document was created with the objective of clarifying and optimizing the use of biological drugs in a disease as complex as SLE. From an analysis of the available evidence, a panel of experts in the management of these patients and in accordance with a preset validated methodology established by consensus has elaborated some recommendations for the use of these agents that can serve as a guide in this matter.
First, it considers that, despite the limitations of the different rates of clinical activity, an effort should be made to achieve their routine application in the care of patients with SLE in order to use them in a more rational way, without incurring in an impairment of the physician's clinical judgment of a particular clinical situation.
BLM (already approved for use in SLE) and RTX are the 2 biologics that currently have, potentially, a greater share of use in SLE. In the first case, its current position seems to be as the first biological agent to be used in cases resistant to standard immunosuppressive therapy who have non-major clinical manifestations, especially in patients with arthritis, cutaneous manifestations and perhaps non-critical vasculitis. The absence of indications in severe cases of nephritis or CNS involvement should not be taken as a contraindication, but rather a lack of data on its use in these cases. For its part, RTX, and regardless of the contradictions above, it seems that it may be useful in patients who fail treatment with BLM and mainly in patients with major clinical manifestations (nephritis, CNS, severe cytopenias) who have not responded to appropriate immunosuppressive drugs.
ABT, TNF-α antagonists and TCZ are very rare options today. Experience with any of these agents is limited and should always take a back seat to the above biological agents unless there is failure or possible intolerance. ABT can be especially effective in cases of arthritis; TNF-α antagonists could be considered in cases of nephritis and TCZ in multiresistant disease seen by laboratory, with experimental evidences that support its use in SLE, but clinical experience that is still scarce and may not establish specific clinical scenarios.
This paper concludes with a description of the future in SLE in the biological drug field, including agents under development, such as epratuzumab, a modulator of B cells, or anti-IFN-α or other agents directed against therapeutic targets, such as the inducible ICOS receptor, IL-10 or IL-17. Today, these agents have no role in clinical practice but are important future prospects for improvement in the treatment of patients with this complex disease.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that this research has not been done in humans or animals.
Confidentiality of dataThe authors state that no patient data appear in this article
Right to privacy and informed consentThe authors state that no patient data appear in this article.
Conflict of InterestMaria Angeles Aguirre Zamorano has attended courses/conferences sponsored by BMS and GSK.
Jaime Calvo Alen has attended courses/conferences sponsored by Abbott, MSD and Pfizer.
Maria Jose Cuadrado has attended conferences sponsored by GSK.
Antonio Fernández Nebro has conducted clinical trials for MSD, Pfizer, Roche and UCB, and has attended courses/conferences sponsored by MSD, Pfizer and Roche. Maria Galindo Izquierdo has received research grants ≥€5000/person/year from GSK and MSD, has conducted clinical trials for Roche and has attended courses/conferences sponsored by Abbott, BMS, GSK, MSD and UCB.
Juan J. Gómez-Reino has received research grants ≥€5000/person/year from BMS, MSD, Roche and UCB, has received speaker fees for ≥€5000/person/year of BMS, MSD, Pfizer, Roche and UCB and has conducted clinical trials for Abbott, BMS, GSK, MSD, Pfizer, Roche and UCB.
Javier Lopez Longo has conducted clinical trials for Roche and UCB, has had contracts with GSK and has attended courses/conferences sponsored by Abbott, BMS, GSK, Roche and UCB.
Estibalitz Loza has received review slides and has attended courses sponsored by Roche.
José Luis Marenco has received honoraria or payments for ≥€5000/person/year from Abbott, Merck, Pfizer and Roche, has spoken at BMS Primary Care events, has conducted clinical trials for Roche and has attended courses/conferences sponsored by BMS, MSD, Pfizer and Roche.
Carmen Martínez Fernández declares no conflict of interest.
Victor M. Martinez Taboada has received research grants ≥€5000/person/year and fees ≥€5000/person/year from Roche, Abbott, has conducted clinical trials for MSD, Pfizer, Roche and UCB, has had contracts with Abbott, Pfizer, Roche, UCB and has attended courses/conferences sponsored by Abbott, BMS, Pfizer, Roche and UCB.
Alejandro Olivo has received research grants, payments and fees for ≥€5000/person/year, has conducted clinical trials and has attended courses/conferences sponsored by Abbott, BMS, MSD, Pfizer and Roche.
José María Pego Reigosa has received research grants ≥€5000/person/year from GSK, Pfizer, Roche and UCB, has spoken at MSD Primary Care events, has conducted clinical trials for UCB and has attended courses/conferences sponsored by Abbott, GSK and Pfizer.
Iñigo Rúa-Figueroa has had contracts with GSK and has attended courses/conferences sponsored by GSK and MSD.
Lucia Silva-Fernandez has attended courses/conferences sponsored by Abbott, Pfizer, Roche and UCB.
Eduardo Angulo Úcar declares no conflict of interest.
Antonio Zea Mendoza has received research grants ≥€5000/person/year of MSD, has conducted clinical trials for Abbott, Merck, Pfizer and Roche, and has attended courses/conferences sponsored by Abbott, BMS, MSD, Pfizer, Roche and UCB.
Please cite this article as: Calvo-Alén J, et al. Consenso de la Sociedad Española de Reumatología sobre el uso de terapias biológicas en el lupus eritematoso sistémico. Reumatol Clin. 2013;9:281–296.