To identify the relationship between serum immunoglobulin levels, complement components 3 and 4, the presence of the HLA-B27 allele and diagnosis of spondyloarthropathies in patients with non-infectious anterior uveitis.
Materials and methodsThe participants were 197 patients with a non-infectious anterior uveitis. The concentrations of serum immunoglobulins, C3 and C4 proteins of the complement were determined by turbidimetry. The personal history of suspected immunodeficiency, ophthalmological complications, arthralgia, family history of spondyloarthropathies and the presence of the HLA-B27 allele were collected.
ResultsA family history of spondyloarthropathy, axial arthralgias, and ophthalmological complications were more frequent in HLA-B27 positive patients (P=.0005, P≤.0001, P≤.0001 respectively) and in patients with spondyloarthropathy diagnoses (P≤.0001, P≤.0001, P≤.0001 respectively). A personal history of recurrent sepsis, and gastrointestinal abnormalities was associated with the presence of the HLA-B27 allele (P≤.0001, P=.0240 respectively) and with the diagnosis of spondyloarthropathy (P=.0492, P=.0017 respectively). IgG decrease was observed (χ2=18.5, OR=5.03, 95% CI=2.32–10.89, P=.0001) and M (OR=7.13, 95% CI=1.40−36.4; P=.0128) in patients positive for the HLA-B27 allele and in patients with a diagnosis of SpA (P=.0364 and P=.0028 respectively). The decrease of C3 proteins (OR=4.82; CI 95%=1.35–17.11; P=.0328) and C4 (OR=9.09; CI 95%=2.13–38.88; P=.0074) were associated with a spondyloarthropathies diagnosis.
ConclusionsPatients with non-infectious anterior uveitis, positive for the HLA-B27 allele and diagnosed with spondyloarthropathies have alterations in serum immunoglobulin levels and complement components 3 and 4, which could contribute to the perpetuation and worse clinical course of this disease.
Identificar la relación entre los niveles séricos de inmunoglobulinas, los componentes 3 y 4 del complemento, la presencia del alelo HLA-B27 y el diagnóstico de espondiloartropatía en pacientes con uveítis anterior no infecciosa.
Materiales y métodosSe incluyeron 197 pacientes con diagnóstico de uveítis anterior no infecciosa. Se determinaron las concentraciones de inmunoglobulinas séricas y proteínas C3 y C4 del complemento mediante turbidimetría. Se recogieron los antecedentes personales de sospecha de inmunodeficiencia, complicaciones oftalmológicas, de artralgias, antecedentes familiares de espondiloartropatías y la presencia del alelo HLA-B27.
ResultadosLos antecedentes familiares de espondiloartropatías, de artralgias axiales y complicaciones oftalmológicas fueron más frecuentes en los pacientes positivos de HLA-B27 (p=0,0005, p≤0,0001, p≤0,0001 respectivamente) y en los pacientes con diagnósticos de espondiloartropatías (p≤0,0001, p≤0,0001, p≤0,0001 respectivamente). Los antecedentes personales de sospecha de inmunodeficiencia, sepsis recurrentes y alteraciones gastrointestinales, se asociaron a la presencia del alelo HLA-B27 (p≤0,0001, y p=0,0240 respectivamente) y al diagnóstico de espondiloartropatía (p=0,0492 y p=0,0017 respectivamente). Se observó disminución de las IgG (χ2=18,5; OR=5,03; IC 95%=2,32−10,89; p=0,0001) y M (OR=7,13; IC 95%=1,40−36,4; p=0,0128) en pacientes positivos del alelo HLA-B27 y en los pacientes con diagnóstico de EspA (p=0,0364 y p=0,0028 respectivamente). La disminución de las proteínas C3 (OR=4,82; IC=1,35−17,11; p=0,0328) y C4 (OR=9,09; IC=2,13–38,88; p=0,0074) se asociaron al diagnóstico de espondiloartropatías.
ConclusionesLos pacientes con uveítis anterior no infecciosa, positivos del alelo HLA-B27 y con diagnóstico de espondiloartropatías tienen alteraciones de los niveles séricos de inmunoglobulinas, los componentes 3 y 4 del complemento, las cuales pudieran contribuir a la perpetuación y peor curso clínico de esta enfermedad.
Anterior uveitis is a group of heterogonous inflammatory eye diseases with complex phenotypes which essentially affect the uveal tract and it is the fifth cause of blindness worldwide.1,2 In Cuba, this disease is considered to be among the primary causes of demand for care in ophthalmologic clinics.3
Spondyloarthropathies (SpA) are chronic inflammatory rheumatic conditions which share many clinical characteristics. These include ankyilosing spondylitis (AS), inflammatory bowel disease, psoriatic arthritis, reactive arthritis, juvenile SpA and juvenile undifferentiated SpA.1,2
SpA are associated with the genes of the major histocompatibility complex, and in particular, with the presence of the human leukocyte antigen-B27 (HLA-B27) allele.3–5 Uveitis is considered to be the most common extra-articular manifestation of SpA and some authors consider that non-infectious anterior uveitis (NIAU) is associated with the HLA-B27 allele within the SpA group.5
Numerous hypotheses have been offered relating infectious processes and other changes to immune response with the triggering and perpetuation of autoimmune and autoinflammatory diseases, which include NIAU and SpA, especially when positivity for the HLA-B27 allele coexists.1,2,4,6
The aim of this study was to identify the relationship between Serum Immunoglobulin Levels, complement Components 3 (C3) and 4 (C4), the presence of HLA-B27 Allele and the diagnosis of SpA in patients with NIAU.
Materials and methodsAn analytical, observational, cross-sectional study was conducted in the period between January 2013 and March 2018.
PatientsPatients who had been diagnosed with NIAU (197) coming from the Cutan Institute of Ophthalmology were included. They were of both sexes, aged 18 years of above and were not receiving immunosuppressant therapy at the time of study inclusion. The patients were assessed by physicians specialising in ophthalmology and immunology. The diagnosis of SpA was obtained from the medical records of the patients and undertaken by a rheumatologist in accordance with the criteria of the Assessment of Spondyloarthritis International Society group, which included AS, psoriatic arthritis, SpA–associated inflammatory bowel disease, reactive arthritis and undifferentiated SpA.7,8 This research study was approved by the ethics committee of the National Centre of Medical Genetics in Cuba. All participants in the research signed an informed consent form. They took into account the ethical principles from the Declaration of Helsinki of the World Medical Associations, for medical research studies on humans.
VariablesDemographic variables were analysed including sex and age. Any background of axial arthralgia (cervical, dorsal spine and sacroiliac joint) and peripheral arthralgia (lower members and hands)9 was recorded, together with familial history of SpA7 and the presence of HLA-B27 allele. Ophthalmologic complications were obtained from the ophthalmologic medical records and included posterior synechias, vasculitis, glaucoma, macular oedema, ocular hypertension, cataracts, retinal detachment, keratitis, hypopion, sclerouveites and decreased visual acuity.1,10 Visual acuity with correction through the LogMAR3 chart. This was defined according to that established by the Standardization of Uveitis Nomenclature-SUN Working Group Standardization of Uveitis Nomenclature for Reporting Clinical Data.11,12
Personal histories were recorded where there was suspicion of immunodeficiency13–15 (Appendix B material supplement, Appendix 1). Serum levels were determined for IgG, IgA and IgM and quantification of components C3 and C4 of the serum complement using a turbidmetry in a chemical analyser (ELIMAT, Italy).
The extraction of deoxyribonucleic acid was made using the saline precipitation technique from 10ml of human blood and the molecular genotype of the HLA-B27 allele using the polymerase chain reaction technique (PTC-200; MJ Research).
Statistical analysisQualitative variables were expressed as frequencies and percentages. The normality of variables was confirmed by the Kolmogorov-Smirnov test. Variables with non-normal distribution were expressed as median and interquartile ranges. The association between study variables with the allele HLA-B27 allele and diagnosis of SpA was identified using the Chi-squared test, odds ratio (OR) as a measurement of association and a 95% confidence interval. The comparison of quantitative variables between groups was measured with the Mann-Whitney U test. Statistical analysis of results was performed with the statistical programme lan 7.0 (Statistica Inc., New York, the United States) and EPIDAT version 3.1 (OPS, Spain). A statistical significance level of .05 was used.
ResultsDemographic and clinical variablesOut of the total of patients with NIAU, 31.5% were HLA-B27 positive, and of these, only 30.6% were diagnosed with SpA. The SpA diagnosis was found in 15.7% of all patients with NIAU (Table 1). The SpA present in patients with NIAU were AS (58.1%), psoriatic arthritis (16,1%), inflammatory bowel disease, (12.9%) and Reiter syndrome (12.9%). 61.3% of patients with SpA tested positive to the HLA-B27 allele. The diagnosis of SpA was associated with the presence of the HLA-B27 allele (OR 4.53; 95% CI 2.0–10.1; P=.0001).
Demographic variables, clinical, serum levels of immunoglobulins and the complement components 3 and 4 according to the presence of the HLA-B27 allele and diagnosis of spondyloarthropathies in patients with non-infectious anterior uveites.
| Variables | Uveitis (n = 197) | Uveites HLA-B27 + (n = 62) | SpA (n = 31) | ||
|---|---|---|---|---|---|
| n (%) | n (%) | OR (95R CI); p1 | n (%) | OR (95R CI); p1 | |
| Male | 81 (41.1) | 29 (46.8) | 1.4 (.8−2,6); .2741 | 19 (61.3) | 4.5 (2,1−10,1); .0001* |
| Age (years), median (IQR); p2 | 46 (34−54) | 45 (34−52); .9324 | 48 (37−54); .2731 | ||
| FH of SpAa | 15 (7.6) | 10 (16.1) | 6.9 (2.0−19.1); .0005* | 8 (25.8) | 7.9 (2.6−23.8); <.0001* |
| Complicationsb | 62 (31.5) | 55 (88.7) | 16.2 (6.8−38.6); <.0001* | 25 (80.6) | 2.4 (8.4-60.0); .0000* |
| Axial arthralgia | 55 (27.9) | 38 (69.1) | 11.0 (5.3−22.6); <.0001* | 14 (82.3) | 45.6 (5.3−174.6); <.0001* |
| Peripheral arthralgia | 32 (12.2) | 27 (84.4) | 20.1 (7.2−55.9); <.0001* | 2 (11.8) | .7 (.1−3.2); 1.0000 |
| Recurrent Sepsis | 71 (36.0) | 38 (61.2) | 4.9 (2.6−9.3); <.0001* | 16 (51.6) | 2.1 (1.0−4.7); .0492* |
| GI changes | 61 (31.0) | 26 (41.9) | 2.1 (1.1−3.9); .0240* | 17 (54.8) | 3.4 (1.5−7.3); .0017* |
| Allergies | 54 (27.4) | 22 (35.4) | 1.8 (.9−3.4); .0852 | 8 (25.8) | .9 (.4−2.3); .8273 |
| IgG reduction | 37 (18.8) | 22 (35.5) | 5.0 (2.2−10.9); .0010* | 10 (27.0) | 2.4 (1.0−5.8); .0364* |
| IgG increase | 5 (2.5) | 0 | NC | 1 (32.2) | 1.3 (.1−12.5); .5793 |
| Decreased IgA | 2 (1.0) | 1 (1.6) | 2.2 (.1−35.7); .5315 | 1 (50.0) | 5.5 (.3−90.3); .2906 |
| Increased IgA | 12 (6.1) | 3 (4.8) | .7 (.19−2.73); .7562 | 1 (8.3) | .5 (.1-3.8); .7507 |
| Decreased IgM | 8 (4.1) | 6 (9.6) | 7.1 (1.40−36.4); .0128* | 5 (17.2) | 10.4 (2.3−46.4); .0028* |
| Increased IgM | 5 (2.5) | 1 (1.6) | .5 (.06−4.90); 1.0000 | 0 | NC |
| Decreased C3 | 7 (3.5) | 4 (6.45) | 3.0 (.66−13.99); .2094 | 4 (14.1) | 4.82 (1.3−17.1); .0328* |
| Increased C3 | 78 (39.6) | 25 (40.3) | 3.0 (.7−134.0); .2094 | 10 (32.3) | 2.20 (.9−5.1); .0595 |
| Decreased C4 | 5 (2.5) | 3 (4.8) | 3.4 (.55−20.8); .1804 | 4 (14.1) | 9.1 (2.1−38.9); .0074* |
| Increased C4 | 34 (17.3) | 11 (17.8) | 1.0 (.4−2.2); 1.000 | 6 (19.3) | 1.9 (.6−5.6); .2524 |
C: Complement serum protein; 95% CI: 95% Confidence Interval; FH: Family History; GI: Gastrointestinal; HLA-B27: Human Leukocyte Antigen B27; Ig: Immunoglobulin; IQR: Interquartile Range; NC: Not calculated by frequency equal to zero; OR: Odds Ratio; p1: Probability of association by χ2; p2: Statistical significance between groups using Mann-Whitney analysis; SpA: Spondyloarthropathis.
A family member with a diagnosis of SpA10: ankylosing spondylitis, psoriatic arthritis, SpA-associated inflammatory bowel disease, reactive arthritis and undifferentiated SpA.
Although there was a predominance of the female sex in patients with uveitis (53.2%), the male sex predominated in those with SpA and this was associated with the SpA diagnosis (P=.0001) (Table 1).
A family history of SpA was associated with the presence of the HLA-B27 allele (P=.0005) in patients with NIAU and with the diagnosis of SpA (P<.0001) (Table 1).
In patients with NIAU axial arthralgias predominated, which were associated with the presence of HLA-B27 (P<.0001), and the diagnosis of SpA (P<.0001) (Table 1).
Ophthalmologic complications were associated with the presence of the HLA-B27 allele (P<.0001) and the diagnosis of SpA (P<.0001) (Table 1). The complications found included: posterior synechias, vasculitis, glaucoma,macular oedema, ocular hypertension, cataracts, retinal detachment, keratitis, hypopion and sclerouveitis.
Personal history of a suspicion of immunodeficiencyRecurrent sepsis (36.0%) was the most common suspected personal history of immunodeficiency which was observed in patients with NIAU. Recurrent respiratory infections (73,2%) and diarrhoeas of infectious aetiology (14.1%) were those most frequent in this group. Recurrent sepsis was associated with the presence of the HLA-B27 allele (P<.0001) and the SpA (p=.0492) (Table 1).
In patients with NIAU, gastrointestinal (GI) alterations were chronic gastritis (73.8%), duodenal or gastric peptic ulcer (27.9%), chronic recurrent diarrhoeas of imprecise aetiology (16.4%) and inflammatory bowel disease (4.9%). These changes were associated with the presence of the HLA-B27 (P=.0240) allele and the SpA (P=.0017) (Table 1).
Allergies were represented by bronchial asthma (75.9%), allergic rhinitis (38.9%) and atopic dermatitis (10%). The allergies were not associated with the presence of the HLA-B27 allele (P=.0852) or with the diagnosis of SpA (P=.8273) (Table 1).
Changes to the humoral immune responseThe reduction of IgG (18.8%) was the most common change of those relating to immunoglobulins in patients with NIAU and was associated with the presence of the HLA-B27 allele (P=.0010), with the diagnosis of SpA (P=.0364) (Table 1), with ophthalmic complications (P=.0002), with recurrent sepsis (P=.0001) and with GI changes (P<.0001) (Table 2).
Association of the decrease of IgG e IgM with sex, ophthalmic complications, arthalgias and personal histories of a suspicion of immunodeficiencies in patients with non-infectious anterior uveites.
| Decrease IgG (n=37) | Decrease IgM (n=8) | ||||
|---|---|---|---|---|---|
| n (%) | OR (95R CI); p1 | Median (IQR); p2 | n (%) | OR (95R CI); p1 | |
| Male | 16 (43.2) | 1.1 (.5−2.3); .7705 | – | 5 (62.5) | 2.5 (.6−10.7); .2772 |
| Age (years) median (IQR); p2 | 45.6 (36−54); .3894 | 37.5 (31.5−54); .6099 | |||
| Complicationsa | 21 (56.8) | 3.8 (1.8−8.0); .0002* | – | 5 (62.5) | 3.9 (.9−16.7); .1116 |
| Arthralgias | 23 (62.2) | 1.9 (.9−3.9); .0937 | – | 5 (62.5) | 1.7 (.4−7.4); .0037* |
| Recurrent sepsis | 17 (64.9) | 4.4 (2.1−10.0); .0001* | – | 7 (87.5) | 2.3 (.6−9.6); .2562 |
| GI alterations | 31 (83.8) | 22.4 (8.6−58.5); <.0001* | – | 4 (50.0) | 2.3 (.6-9.6); .2562 |
| Allergies | 13 (35.1) | 1.6 (.7−3.4); .2425 | – | 3 (37.5) | 1.9 (.4−8.1); .4140 |
95R CI: 95% Confidence Interval; GI: Gastrointestinal; Ig: Inmunoglobulin; IQR: Interquartile Range; OR: Odds Ratio; p1: Statistical significance for the association through χ2; p2: Statistical significance between groups using Mann-Whitney analysis.
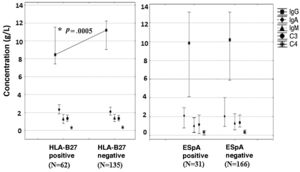
In patients with positive NIAU HLA-B27 lower serum values of IgG (U=1.979,5; P=.0005) were observed, with a median of 8.4g/l (7.4–11.5), whilst in patients with negative NIAU HLA-B27 the median was 11.2g/l (9.0–12.2) (Fig. 1).
Serum concentration of immunoglobulins and proteins of the complement according to the presence of the HLA-B27 allele and diagnosis of spondyloarthropathy.
C: complement; SpA: spondyloarthropathy; HLA-B27: human leukocyte antigen B27; Ig: immunoglobulin.
* Significant differences using the Mann-Whitney U test among HLA-B27 positive and negative patients (P<.05).
The reduction of IgM was associated with the presence of the HLA-B27 allele (P=.0028), with the diagnosis of SpA (P<.0001) and the presence of arthralgias (P=.0037) (Table 1).
The reduction of at least one of the immunoglobulins studied was presented in 42 patients (21.3%) (Table 3).
Immunoglobulin alterations in patients with diagnosis of non-infectious anterior uveitis.
| Decrease | Increase | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgM | IgA | IgG IgM | IgG IgA | IgA IgM | Total | IgG | IgM | IgA | IgG IgM | IgG IgA | IgA IgM | Total | |
| No. of patients | 33 | 5 | – | 3 | 1 | – | 42 | 3 | 1 | 10 | 2 | – | 2 | 18 |
| Percentage | 16.8 | 2.5 | – | 1.5 | .5 | – | 21.3 | 1.5 | .5 | 5.1 | 1.0 | – | 1.0 | 9.1 |
Ig: Immunoglobulins.
Of the changes to complement in patients with NIAU an increase in C3 (39.6%) predominated. However, there was an association of the decrease of C3 (OR 4.82; 95% CI 1.35–17.11; P=.0328) and a decrease of C4 (OR 9.09; 95% CI 2.13–38.88; P=.0074) with the diagnosis of SpA. No association of the increase of C3 and C4 was observed with the variables analysed in the study (Tables 1 and 4).
Association of drop in C3 and C4 with sex, ophthalmologic complications, arthralgias and personal backgorund of suspicion of immunodeficiency in patients with non-infectious anterior uveitis.
| Decrease C3 | Increase C3 | Decrease C4 | Increase C4 | |||||
|---|---|---|---|---|---|---|---|---|
| n (%) | OR (IC); p1 | n (%) | OR (IC); p1 | n (%) | OR (IC); p1 | n (%) | OR (IC); p1 | |
| Male | 2 (28.6) | .6 (.1−3.0); .7022 | 40 (51.3) | 2.0 (1.1−3.6); .0189* | 4 (80.0) | 6.0 (.6−54.5); .1613 | 13 (38.2) | .9 (.4−1.8); .7074 |
| Age (years). median (IQR); p2 | 51 (32−62); .4873 | 44 (34−53); .2844 | 56 (51−62); .1224 | 48 (34−52); .5166 | ||||
| Complicationsa | 4 (57.1) | 3.1 (.7−14.2); .2073 | 25 (32.0) | 1.1 (.6−2.08); .8266 | 2 (40) | 1.5 (.2−9.2); .6464 | 14 (41.2) | 1.7 (.8−3.6); .1659 |
| Arthralgias | 3 (42.9) | .7 (.2-3.4); 1.0000 | 39 (50.0) | 1.0 (.6-1.8); .9540 | 3 (60.0) | 1.5 (.2−9.3); .6827 | 17 (50.0) | 1.0 (.5−2.1); .9740 |
| Recurrent sepsis | 2 (28.6) | .7 (.1−3.7); 1.0000 | 30 (38.5) | 1.2 (.7−2.1); .5667 | 3 (60.0) | 2.7 (.4−16.8); .3532 | 14 (41.2) | 1.3 (.6−2.8); .4929 |
| GI changes | 3 (42.9) | 1.7 (.4−7.9); .6789 | 22 (28.2) | .9 (.4−1.5); .4977 | 2 (40.0) | 1.5 (.2−9.1); .6489 | 8 (23.5) | .6 (.3−1.5); .3026 |
| Allergies | 2 (28.6) | 1.2 (.2−6.4); 1.0000 | 20 (25.5) | 1.1 (.6−2.0); .8469 | 3 (60.0) | 4.76 (.8−29.3); .0987 | 11 (32.3) | 1.6 (.7−3.4); .2845 |
C: Complement serum protein; 95R CI: 95% Confidence Interval; GI: Gastrointestinal; IQR: interquartile range; OR: Odds Ratio; p1: Statistical significance for the association through χ2; p2: Statistical significance between groups using Mann-Whitney analysis.
Anterior uveitis is a health problem because it is one of the most common causes of blindness worldwide.4–6
HLA-B27 allele in patients with NIAU and SpAThe percentage of patients positive for HLA-B27 with NIAU are within the range described by other authors, which may vary from 12% to 88%, depending on the geographical area of the study population.1,2,13
The expression of the HLA-B27 allele is insufficient in itself for presenting NIAU or SpA.4 However, several approaches try to explain the relationship between the allele studied and NIAU. The molecular mimicry and the arthritogenic peptide hypothesis considers that these diseases are autoimmune. The hypothesis of incorrect folding of molecules HLA-B27 and the deposit of β2 macroglobulin chains on synovial tissues include them within the spectrum of autoinflammatory diseases.4,16
The hypothesis of an abnormal HLA-B27 molecule folding is based on the ability of the heavy chains of these molecules to form stable homodimers. These are retained and accumulated in the endoplasmic reticulum which constitutes a response to stress and chronic inflammation.4,16
In Cuba the diagnosis of SpA in patients with NIAU was described in a range from 10% to 38%, similar to that observed in other studies.4,5
The percentage of patients with a diagnosis of SpA and the presence of the allele studied was compared with those found in other studies, which were between 40% and 90%. NIAU may be present on diagnosis of SpA, may be a complication in the course of this disease or precede clinical manifestations of SpA,5 which could explain the low prevalence of SpA in patients with positive NIAU for HLA-B27 in this study. Other authors have reported that the SpA is not the most prevalent of aetiologies in patients with NIAU.17,18
Furthermore, many patients with positive NIAU for HLA-B27 who fulfil the clincial and radiological criteria of SpA are not diganosed.5,19
NIAU associated with the presence of this allele is a requisite for suspecting the existence of undiagnosed SpA during long periods of time, which has a poorer prognosis.5,20
The greater number of patients with a diagnosis of SpA were HLA-B27 positive. The prevalence of SpA in patients with NIAU HLA-B27 positive is higher than 50% in most of the studies.1,2,4,6
The presence of the HLA-B27 allele has been associated with a greater frequency of AS, as occurred in this research.1,4,5
Demographic and clinical characteristicsPrevious studies record a preponderance of the female sex in patients with NIAU.1
Other authors also reported a preponderance of the male sex in patients with SpA.2,5 The most common extra-articular manifestation in male patients with SpA is NIAU, whilst in female patients it is psoriasis and inflammatory bowel disease. It has been suggested that in many cases this leads to a delayed diagnosis of SpA in women and a preponderance of males in this disease, which also explains why men have more severe radiologic conditions. Furthermore, it is known that differences exist between both sexes regarding immunological, hormonal and genetic responses.21
Previous studies report on medians of similar age to those of this research (6). NIAU occurs in people who are professionally active.2,3
Both a family history of SpA and a personal background of arthralgias constitute diagnostic SpA criteria,7,8 which fits in with the association of these histories both with the diagnosis of SpA and with the presence of the HLA-B27 allele in this study.
Other authors found there was predominance in the reduction of visual acuity in patients with NIAU and HLA-B27 positives, as occurred in this research.10,22
Personal history of a suspicion of immunodeficiencyOne of the most important findings from this research was the relationship between personal histories of a suspicion of immunodeficiency of recurrent sepsis and GI alterations with the presence of the HLA-B27 allele and the SpA diagnosis.
A previous history of infection or pore oral hygiene has been associated with the development of NIAU, a fact which has led to the support for the systemic infection model as a potential trigger of this disease in humans.1,23
An analysis study of key genes and pathways of inflammatory uptake found that in patients with NIAU and positive HLA-B27, changes existed in the metabolic signal pathways, of chemokines, and in the cytosine interaction of the receptors and processes involved in response to infections.2,6
The hypothesis of molecular mimicry and arthrithogenic peptide, however, suggests that the division of peptide from the HLA-B27 molecule may present microbial peptides similar to certain antigens, which allows the immune response of the positive lymphocytes T CD8 (Cluster of differentiation), restricted by the molecules HLA-B27 against microbial antigens to react with arthrogenic peptides and trigger chronic inflammation which is characteristic of this disease. These T CD8 s+ activated cells induce inflammation as a result of cross-over reactivity between exogenous antigens and peptides located in the eye.1,7
It has been suggested that inflammatory stimuli such as infectious processes are able to provoke the rupture of the retinal-blood supply barrier and aid migration of T-cells, which recognise their own antigens (retinal antigens) as foreign or react to them through molecular mimicry and trigger uveitis.2,4 Several authors consider the role of chronic infection by Helicobacter pylorie to be major in ocular diseases such as glaucoma, mucous tissue-associated lymphoma, blepharitis, central serous chorioretinopathy and anterior uveitis.24
The raised frequency of chronic gastritis and peptic ulcer (duodenal or gastric) found in the study, could be associated with infection by Helicobacter pylori and giardiasis. Patients with impaired antibody response are more likely to suffer from autoimmune or autoinflammatory diseases such as uveitis.15 Other resarchers observed infection by Helicobacter pylori in patients with anterior uveitis and ocular hypertension.6,8
Chronic diarrhoeas of unspecified aetiology and recurrent sores may form part of the clinical symptoms of an inflammatory bowel disease which was undiagnosed or the onset of which began at the time of study.5 A small group of patients also presented with a diagnosis of inflammatory bowel disease. These elements may contribute to the association of GI changes with the presence of the allele studied and the SpA diagnosis.
Although allergies were not associated with changes to the humoral immune response, they predispose the body to recurrent infections due to propitiating in an inflamed tissue the invasion of infectious agents. Immune deficiencies also determine hypersensitive or allergic processes, due to changes in immune responses.13,14
Changes in the humoral immune responseOther important findings from this research were the association between the reduction of IgG and IgM and the presence of HLA-B27, and also between the reduction of IgG and IgM and the diagnosis of SpA.
Several authors have published that one of the causes of anterior uveitis is the presence of common variable immunodeficiency, which is mostly determined by the reduction of IgG.6,14 However, the patients with changes in immune response by immunodeficiency have a greater predisposition to developing autoimmune and autoinflammatory processes.3,14,25,26
Notwithstanding it was found that other authors observed hypergammglobulinaemia and an increase in circulating immunocomplexes in patients with uveitis, with no evidence of their relationship with the clinical evolution or pathogenesis of the disease. An increase in some immunoglobulins was also been found in the study, such as that of IgA, which could correspond to a response to recurrent infections in some patients. The association of reduction of IgG with positive NIAU HLA-B27, SpA, complications, recurrent sepsis and GI changes may be explained from the reduction of immunoglobulins being predisposed to infectious processes, which in turn perpetuate and aggravate the inflammatory processes and also predispose them to other autoimmune and autoinflammatory diseases.4,13,15,16
As SpA are autoinflammatory or autoimmune diseases, they may require treatments with immunosuppressants, which lead to a reduction of immunoglobulins.14,15
In this research it was not possible to establish a definitive diagnosis of primary immunodeficiency due to the fact that assessment of antibody response to vaccine antigens is necessary, together with assessment of number and function of T cells and molecular (9) biology studies. This was one of the study limits. One element to consider for diagnosis of primary immunodeficiency was to rule out the presence of secondary immunodeficiencies, such as those from immunosuppressant treatments, which are needed on occasions in SpA, and in several patients with NIAU.15
Although the association found between the reduction of complement serum proteins C3 and C4 and the diagnosis of SpA was of note, their role in NIAU remains controversial.
In one genetic analysis study of the complement protein C3 no risk of susceptibility to uveitis was found.1,4 However, it was published that with SpA patients who presented with a reduction in complement proteins and antibodies suffered from greater complications and a severe disease course which may be explained by the effects of the recurrent infections in these patients. The patients with defects of these proteins exhibit the two-directional interaction complex between immunodeficiencies and autoimmune diseases.26
ConclusionsPatients with NIAU, positive for the HLA-B27 allele and with a diagnosis of SpA have changes to serum levels of immunoglobulins and to components C3 and C4 of the complement, which may contribute to the perpetuation and poorer medical course of this disease.
FinancingThis study was financed by the Cuban Ministry of Public Health.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Torres Rives B, Martínez Téllez G, Mataran Valdés M, Collazo Mesa T, Colás González R, Frutos Ambou I. Niveles séricos de inmunoglobulinas, componentes 3 y 4 del complemento, alelo HLA-B27 y espondiloartropatía en pacientes con uveítis anterior no infecciosa. Reumatol Clin. 2021;17:575–581.