Rheumatoid arthritis (RA) patients with disease in clinical remission might show subclinical synovitis, which can be related to the progress of structural joint damage.
ObjectiveTo determine and compare the degree of synovial inflammation by ultrasound (US) in patients with RA in clinical remission, treated with DMARD or combination therapy with DMARD and anti-TNF.
MethodsHospital-based cross-sectional study of 58 patients with RA in sustained remission for at least 6 months by DAS28 <2.6, who attended the Rheumatology Service at the Hospital Universitario de Caracas. Patients underwent clinical, functional, and laboratory assessments. Ultrasound was performed in hands measuring synovial effusion, synovial hypertrophy and power Doppler signal; using a semiquantitative 4-point scale of 0=none to 3=severe. Chi-square and t-test were used to compare the clinical, functional, laboratory and US assessments between the DMARD (N=37) and combination therapy with DMARD and anti-TNF (N=21) groups. A p-value <0.05 was considered statistically significant.
ResultsOut of 58 patients, 25.9% had remission by US and 74.1% had synovial effusion or hypertrophy or positive power Doppler signal. Non-significant differences in US synovitis between the two groups were found.
ConclusionsPersistent US activity was evident in a high percentage of rheumatoid arthritis patients in clinical remission by DAS28. No differences in subclinical synovitis measured by US were found between patients with DMARD and anti-TNF-induced clinical remission.
Los pacientes con artritis reumatoide (AR) con enfermedad en remisión clínica pueden mostrar sinovitis subclínica, que puede estar relacionada con el progreso del daño articular estructural.
ObjetivosDeterminar y comparar el grado de inflamación sinovial medida por ultrasonido (US) en pacientes con AR en remisión clínica, tratados con FAME o FAME y anti-TNF.
MétodosEstudio transversal con sede en hospital, donde se evaluaron 58 pacientes con AR en remisión sostenida durante al menos 6 meses por DAS28<2,6, que asistieron al Servicio de Reumatología del Hospital Universitario de Caracas. Se realizó evaluación clínica, funcional y de laboratorio. Se practicó US en manos evaluando derrame, hipertrofia sinovial y presencia de señal power Doppler, utilizando una escala semicuantitativa de 4 puntos 0=ninguno a 3=severo. Se compararon los hallazgos entre los pacientes con FAME (n=37) y FAME y anti-TNF (n=21), utilizando las pruebas de Chi-cuadrado y t de Student. Se consideró significación estadística si el valor de p era<0,05.
ResultadosDe los 58 pacientes, el 25,9% tuvo remisión por US y el 74,1% presentó derrame, hipertrofia sinovial o señal power Doppler positiva. No hubo diferencias significativas en la presencia de sinovitis medida por US entre los 2 grupos.
ConclusionesEn pacientes en remisión clínica por DAS28, la actividad persistente medida por US fue evidente en un alto porcentaje. No hubo diferencias en la sinovitis subclínica medida por US entre los pacientes en remisión clínica inducida con FAME y FAME y anti-TNF.
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation of the synovial membrane or synovitis that causes destruction of the intra-articular and periarticular structures.1 Modern treatment for RA has helped many patients achieve higher levels of clinical remission.2 However, the progress of joint damage has been shown to be associated with subclinical inflammation as measured by magnetic resonance imaging (MRI) and ultrasound (US).3,4 US synovitis has been assessed by gray scale and power Doppler, with power Doppler positive signal indicating active synovitis.5
Several studies have shown subclinical inflammation by MRI and US.3–7 For example, Brown et al.6 found, in RA patients in clinical remission treated with disease-modifying antirheumatic drugs (DMARD), that 92.6% had synovitis and 46.4% had bone edema as shown by MRI; and US showed that 73% had gray-scale synovial hypertrophy and 43% had increased power Doppler signal. Saleem et al.7 studied 100 RA patients with disease remission, half induced by DMARD and half induced by the combination of TNF blockade and methotrexate, to determine whether the difference in radiographic outcome results from more complete suppression of imaging detecting synovitis. They found that the proportion of patients in remission did not differ on imaging (16% for DMARD vs 10% for combination therapy). However, the combination treatment group had more gray scale synovitis but similar power Doppler activity (60% DMARD vs 48% combination therapy).
Because patients with disease in clinical remission might show subclinical synovitis, we assessed whether DMARD and anti-TNFα therapy induced clinical remission differed by US. The objectives of this study were (1) to determine and compare the degree of synovial inflammation (subclinical activity) by gray scale and power Doppler signal on US in patients with RA in clinical remission, treated with DMARD or combination therapy with DMARD and anti-TNFα; and (2) to examine the correlation between clinical and biological variables with US findings.
Patients and MethodsIn this hospital-based cross-sectional study, 58 patients with RA who attended the Rheumatology Service at the Hospital Universitario de Caracas were assessed. Inclusion criteria were: 1. Fulfilling the 1987 American College of Rheumatology criteria for RA8; and 2. Sustained clinical remission induced by DMARD or by DMARD and anti TNFα (combination therapy) for at least six months, defined as a Disease Activity Score 28 joints (DAS28) below 2.6.9 Patients underwent clinical, Health Assessment Questionnaire (HAQ),10 laboratory, and musculoskeletal US assessments. All patients signed inform consent and the study was approved by the Ethical Committee of the institution. A rheumatologist performed the clinical evaluation and two rheumatologist trained in musculoskeletal US (which were blinded to clinical and laboratory data) performed the US assessment.
A My Lab 25 ESAOTE US machine with a 10–18MHz linear transducer was used to perform US assessment. The gray scale and power Doppler parameters were standardized between sonographers and the pulse repetition frequency for power Doppler was adjusted to 0.7 to maximize sensitivity. To measure inter-observer variability prior to the inclusion of patients, 30 patient images with RA were randomly selected from an image bank and assessed by Sonographer 1 (MC) and Sonographer 2 (JC) for the presence/absence of synovial effusion, synovial hypertrophy, and power Doppler signal. The Kappa coefficient for the inter-observer variability between Sonographer 1 and Sonographer 2 was 0.61, 0.52, and 1.00, respectively (good, moderate, and excellent agreement). Six months later, these 30 images of patients with RA were reassessed for the intra-observer variability. The Kappa coefficient for synovial effusion and synovial hypertrophy was 0.74 and 0.8, respectively (good agreement) for Sonographer 1. The Kappa coefficient for synovial effusion and synovial hypertrophy was 0.67 and 0.92, respectively, for Sonographer 2 (good and excellent agreement). There is no international agreement about which joints should be assessed by US to measure subclinical activity in patients with RA. US was performed in the hands (wrist, 2nd to 5th MCF, and 2nd to 5th IFP, bilateral) following the technique described by Backhaus et al.,11 assessing effusion and synovial hypertrophy by gray scale US and power Doppler signal (OMERACT definition).12 To quantify synovial effusion and synovial hypertrophy, a validated semiquantitative scale was used, where 0=none and 3=severe.13 To quantify power Doppler signal (active synovitis), a validated semiquantitative scale was used, where 0=absent (without synovial flow), 1=mild (≤3 isolated signals), 2=moderate (≥3 isolated signals or confluent signals in less than half of the synovial area), and 3=severe (signals in more than half of the synovial area).13 Eighteen joints in 58 patients (n=1040 joints) were evaluated. A total score of gray scale and power Doppler signal (the addition of synovial effusion, synovial hypertrophy and power Doppler signal scores of each evaluated joint) for each patient was calculated. There is no consensus about the definition of US remission in RA patients. We considered US remission a total US score of gray scale and power Doppler signal equal to 0. Demographic, clinical, functional, biological, and musculoskeletal US were compared between patients with DMARD and those with combination therapy.
Statistical Analysist-Test was used for variables with normal distribution, Wilcoxon–Mann–Whitney for variables with non-normal distribution, and Chi-square or Fisher Exact tests for categorical variables. Correlations between clinical, HAQ, biological, and US variables were performed using the Spearman correlation test. For all analyses, a two-tailed approach was used and statistical significance was assigned when type I error probability was equal to or less than 5% (p<0.05). All analyses were performed using the SAS System for Windows, version 9.4 (SAS Institute, Inc., Cary, NC).
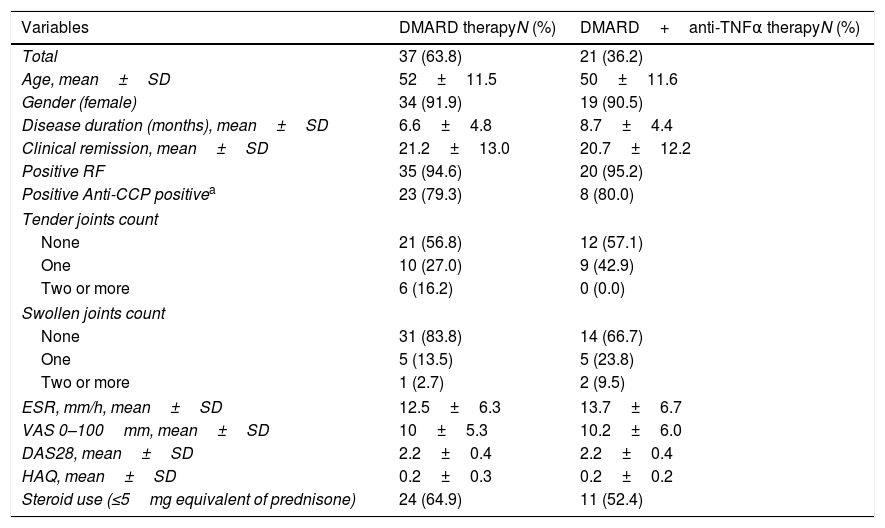
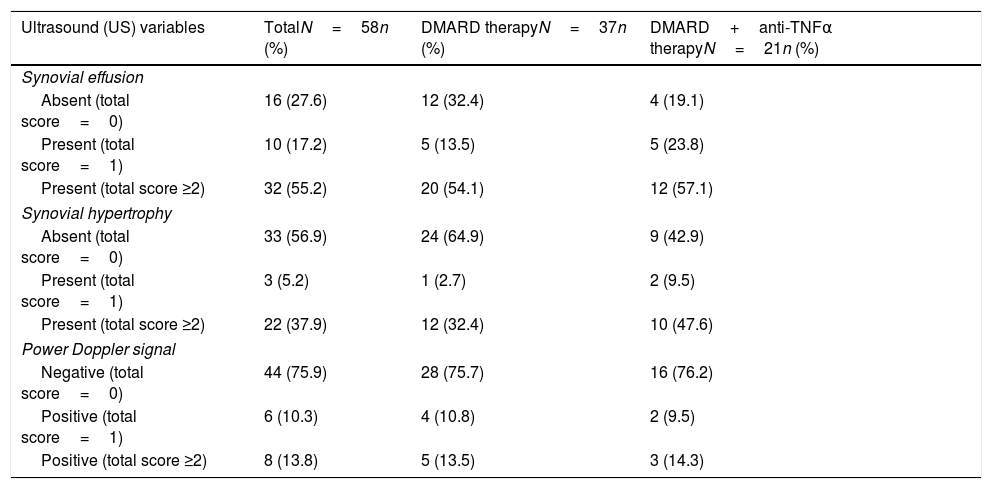
ResultsThe mean age was 52±11.45 years and 91% were women. The average disease duration was 7±4.8 years and the average month in clinical remission was 21±12.6. Out of 58 patients, 37 were in the DMARD group and 21 in the combination therapy group. Ninety-five percent had rheumatoid factor positive. Anti-CCP was performed in 39 patients and was positive in 79% of the cases. The mean DAS28 was 2.2±0.4 and the mean HAQ was 0.24±0.3. One hundred percent of the 58 patients received DMARD, of whom 86% (n=50) received methotrexate alone or in combination with another DMARD or biologic therapy (81% of combination therapy group received MTX). Non-significant differences in demographics, clinical, physical function, and biological variables were found between the two groups (Table 1). Only 25.9% had remission by US and 74.1% had synovial effusion or hypertrophy or positive power Doppler signal. Table 2 presents synovitis detected by US in patients with DMARD and combination therapy. In total, 72.4% had synovial effusion, 43.1% synovial hypertrophy, and 24.1% positive power Doppler signal. In the DMARD group (n=37), 67.6% had synovial effusion, 35.1% synovial hypertrophy, and 24.3% positive power Doppler signal. In the combination therapy group (n=21), 80.9% had synovial effusion, 57.1% synovial hypertrophy, and 23.8% positive power Doppler signal. Non-significant differences in the US variables were found between the two groups.
Demographic, Clinical, and Biological Characteristics of the Sample by Treatment Groups (DMARD and DMARD Plus Biological Therapy) (N=58).
| Variables | DMARD therapyN (%) | DMARD+anti-TNFα therapyN (%) |
|---|---|---|
| Total | 37 (63.8) | 21 (36.2) |
| Age, mean±SD | 52±11.5 | 50±11.6 |
| Gender (female) | 34 (91.9) | 19 (90.5) |
| Disease duration (months), mean±SD | 6.6±4.8 | 8.7±4.4 |
| Clinical remission, mean±SD | 21.2±13.0 | 20.7±12.2 |
| Positive RF | 35 (94.6) | 20 (95.2) |
| Positive Anti-CCP positivea | 23 (79.3) | 8 (80.0) |
| Tender joints count | ||
| None | 21 (56.8) | 12 (57.1) |
| One | 10 (27.0) | 9 (42.9) |
| Two or more | 6 (16.2) | 0 (0.0) |
| Swollen joints count | ||
| None | 31 (83.8) | 14 (66.7) |
| One | 5 (13.5) | 5 (23.8) |
| Two or more | 1 (2.7) | 2 (9.5) |
| ESR, mm/h, mean±SD | 12.5±6.3 | 13.7±6.7 |
| VAS 0–100mm, mean±SD | 10±5.3 | 10.2±6.0 |
| DAS28, mean±SD | 2.2±0.4 | 2.2±0.4 |
| HAQ, mean±SD | 0.2±0.3 | 0.2±0.2 |
| Steroid use (≤5mg equivalent of prednisone) | 24 (64.9) | 11 (52.4) |
Note: Performed in 39 patients; DMARD=disease-modifying antirheumatic drugs; RF=rheumatoid factor; Anti-CCP=cyclic citrullinated peptide antibody; ESR=erythrocyte sedimentation rate; VAS=Visual Analog Scale Pain; DAS-28=Disease Activity Score 28-joint assessment; HAQ=Health Assessment Questionnaire.
Synovitis Detected by US in Patients With Disease-modifying Antirheumatic Drugs (DMARD) and DMARD Plus Biological Therapy (N=58).
| Ultrasound (US) variables | TotalN=58n (%) | DMARD therapyN=37n (%) | DMARD+anti-TNFα therapyN=21n (%) |
|---|---|---|---|
| Synovial effusion | |||
| Absent (total score=0) | 16 (27.6) | 12 (32.4) | 4 (19.1) |
| Present (total score=1) | 10 (17.2) | 5 (13.5) | 5 (23.8) |
| Present (total score ≥2) | 32 (55.2) | 20 (54.1) | 12 (57.1) |
| Synovial hypertrophy | |||
| Absent (total score=0) | 33 (56.9) | 24 (64.9) | 9 (42.9) |
| Present (total score=1) | 3 (5.2) | 1 (2.7) | 2 (9.5) |
| Present (total score ≥2) | 22 (37.9) | 12 (32.4) | 10 (47.6) |
| Power Doppler signal | |||
| Negative (total score=0) | 44 (75.9) | 28 (75.7) | 16 (76.2) |
| Positive (total score=1) | 6 (10.3) | 4 (10.8) | 2 (9.5) |
| Positive (total score ≥2) | 8 (13.8) | 5 (13.5) | 3 (14.3) |
Note: DMARD=disease-modifying antirheumatic drugs.
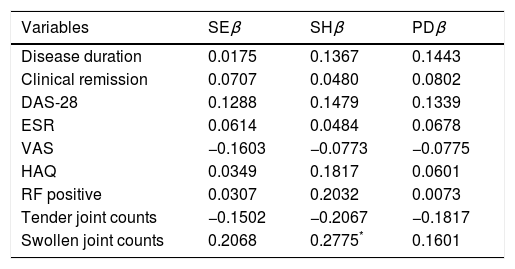
Correlation between clinical, HAQ, and biological variables with US findings (Table 3) showed a statistically significant positive correlation only between the number of swollen joints and synovial hypertrophy assessed by US (correlation coefficient=0.28).
Correlation Between Clinical and Biological Variables With US Findings.
| Variables | SEβ | SHβ | PDβ |
|---|---|---|---|
| Disease duration | 0.0175 | 0.1367 | 0.1443 |
| Clinical remission | 0.0707 | 0.0480 | 0.0802 |
| DAS-28 | 0.1288 | 0.1479 | 0.1339 |
| ESR | 0.0614 | 0.0484 | 0.0678 |
| VAS | −0.1603 | −0.0773 | −0.0775 |
| HAQ | 0.0349 | 0.1817 | 0.0601 |
| RF positive | 0.0307 | 0.2032 | 0.0073 |
| Tender joint counts | −0.1502 | −0.2067 | −0.1817 |
| Swollen joint counts | 0.2068 | 0.2775* | 0.1601 |
Note: US=ultrasound; SE=synovial effusion; SH=synovial hypertrophy; PD=power Doppler; DAS-28=Disease Activity Score 28-joint assessment; ESR=erythrocyte sedimentation rate; VAS=Visual Analog Scale Pain; HAQ=Health Assessment Questionnaire; RF=rheumatoid factor; β=coefficient.
This study evaluated and compared the degree of synovial inflammation (subclinical activity) by gray scale and power Doppler signals on US in patients with RA in clinical remission, treated with DMARD or combination therapy with DMARD and anti-TNF. Synovitis was detected using US gray scale and power Doppler signal in a high percentage of the patients, which implies that they are at high risk of disease progression and structural joint damage. Several studies have shown that sustained inflammation, measured by US, has been associated not only with progression of joint damage but also with relapses.3–6,14 This association is very important for clinicians treating RA patients, because these results suggest that US should be a part of the evaluation of patients with RA in clinical remission, to detect those at risk of joint damage and consider adjusting their therapy appropriately.
We did not find significant differences in the percentages of synovial effusion, synovial hypertrophy, and power Doppler signal between patients with DMARD and those with combination therapy. Our results are similar to the study of Saleem et al., although they reported a higher gray scale synovitis in the combination therapy group (a group with a longer duration of disease).7 This could mean that the degree of subclinical inflammation is independent of the treatment used to achieve clinical remission. Although clinical remission is achieved, US assessment is important to determine which patients are at high risk of joint damage.
The evaluation of correlations between clinical and biological variables with US variables showed a positive correlation between the number of swollen joints and synovial hypertrophy assessed by US. Patients in this study had established disease and the persistency of joint inflammation could correlate with thickening of the synovial tissue, allowing the US to detect joint damage and through the positive power Doppler signal to detect active synovitis.
This study has some limitations. First, the use of DAS28 to classify patients in clinical remission allows patients with few painful and/or swollen joints remain in the range of remission and does not evaluate joints from the feet, which can mistakenly classify patients with mild or moderate activity. The Simple Disease Activity Index (SDAI) and the ACR/EULAR definition of remission reported in 2011 could represent more accurate indices to assess remission in patients evaluated by US.15,16 However, the DAS28 is widely used in most studies to assess RA activity and is the index used by the Rheumatology Service of our hospital. Second, low-grade power Doppler and gray scale ultrasound signals may not necessarily reflect the presence of active synovitis in RA joints.17 Indeed, 11% of the joints of healthy volunteers showed power Doppler signals for joint activity,18 likely corresponding to the presence of physiologic vessels. A score of gray scale or power Doppler signal ≥2 in a given joint is a more reliable reflection of ongoing inflammation in a state of clinical remission.17 Third, the joints selected to assess disease activity by US did not include all 28 joints in DAS28; however, other studies have evaluated these joints in RA patients to define clinical remission. These joints are the most commonly affected in RA patients.3,5,7,16 Moreover, in a study by Naredo et al.,19 the assessment of these joints showed a good correlation when compared with the assessment of 44 joints by US. However, these authors reported a better correlation with a bilateral evaluation of wrists (2nd to 5th MCFs), ankles (2nd to 5th MTFs), and the evaluation of 12 joints (elbow, wrist, 2nd and 3rd MCF, knee and ankle bilateral).
Lastly, we did not have a control group, the study included a convenient sample from a single setting, and the findings cannot be generalized to all patients with RA in Venezuela. Despite all these limitations, this is the first study conducted in the country to analyze subclinical activity by gray scale and power Doppler signal on US in patients with RA in clinical remission. We can conclude that, in RA patients in clinical remission measured by the DAS28, the persistency of US subclinical activity was high. US assessment has been shown to be a useful tool to determine subclinical activity in patients with clinical remission and to identify patients at increased risk of progression of joint damage and relapses, which could have implications for therapeutic decisions. No differences in the assessment of subclinical activity were found between patients whose clinical remission was caused by DMARD or combination therapy. Routinely use of US in clinical practice to evaluate RA patients in clinical remission is recommended to monitor subclinical activity.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of InterestThe authors declare that they have no conflicts of interest.
We thank the Division of Rheumatology at the Hospital Universitario de Caracas for their support in carrying out this study and Abbott Laboratories of Venezuela, for the support provided through the donation to the Division of Rheumatology of the My Lab 25 Ultrasound (Esaote Biomedica, Genoa, Italy). The authors acknowledge the assistance of Sarah Toombs Smith, PhD, ELS, in editing the manuscript.