Spondyloarthropathies (SpA) are disabling diseases with a prevalence of 1.9% in the general population. The indices designed for monitoring the disease should be valid, reliable and cross-culturally adapted for decision-making concerning the appropriate treatment. Changing an adjective or pronoun in a self-administered questionnaire could be the big difference in condensing an idea in a few words and transmitting that concept to all those who share the same language.
ObjectivesTo develop a Venezuelan version of the original English version of the BASDAI/BASFI and to evaluate its reliability and validity in Venezuelan patients with SpA.
MethodsCertified linguists were needed for the translation of a Venezuelan version of the BASDAI/BASFI. The evaluation of reliability and validity was performed by calculating correlation coefficients in addition to Cronbach's alpha correlation between the BASDAI score and the clinical parameters (for example: erythrocyte sedimentation rate, C-reactive protein, modified Schöber test, occiput-to-wall distance and enthesis count).
ResultsWe studied 40 patients including 31 men (77.5%) and 9 women (22.5%). The mean age was 35.9 years±standard deviation (SD) 12.01 and the disease duration was 11.5 years (±SD 9.5). The most common diagnoses were undifferentiated spondyloarthritis (45%), ankylosing spondylitis (27.5%) and psoriatic arthritis (20%). The incidences of reactive arthritis, ankylosing spondylitis and juvenile Reiter's syndrome were 2.5% each. The test–retest reliability of the BASDAI and BASFI was high (R=0.99 and 0.99, respectively; P<.0001). The internal consistency for the BASDAI was high (Cronbach's alpha=0.88; P=.002) and the intraclass correlation coefficient for internal consistency: 0.9867 (P=.001). Internal consistency for the BASFI: Cronbach's alpha=0.7985 (P=.002), intraclass correlation coefficient for internal consistency: 0.9055 (P=.001).
Construct validity of the BASDAI was high for general well-being of the patient (R=0.84) and for enthesis count (R=0.84). Low back pain showed moderate correlation with BASDAI (R=0.69; P<.0001) and the erythrocyte sedimentation rate showed a low correlation (R=0.39683; P=.0112).
ConclusionThe Venezuelan version of the BASDAI/BASFI could be used in clinical research to assess and evaluate the course of disease activity in Venezuelan SpA patients.
Las espondiloartritis (EsA) son enfermedades discapacitantes, con una prevalencia del 1,9% en la población general; los índices diseñados para su seguimiento deben ser válidos, confiables y adaptados transculturalmente para decidir el tratamiento y la vigilancia adecuada. El cambiar un adjetivo o un pronombre en un cuestionario autoadministrado puede ser la gran diferencia cuando se quiere investigar el estado actual de la enfermedad.
ObjetivosDesarrollar una versión venezolana de la versión original en inglés de BASDAI/BASFI, para evaluar su confiabilidad y validez en pacientes venezolanos con EsA.
MétodosSe necesitaron lingüistas certificados para la traducción de una versión venezolana de BASDAI/BASFI. La evaluación de la confiabilidad y la validez se realizó mediante el cálculo de coeficientes de correlación, además de la correlación α de Cronbach, entre la puntuación de BASDAI y los parámetros clínicos (p. ej., ESR, CRP, índice de prueba de Schöber modificado, distancia occipucio-pared y recuento de entesis).
ResultadosCuarenta pacientes, 31 hombres (77,5%) y 9 mujeres (22,5%), con una edad promedio ± desviación estándar 35,9±12,0 años. Duración de la enfermedad fue de 11,5±9,5 años. El 45% presentaba EsA indiferenciada, el 27,5% espondilitis anquilosante y el 20,0% artritis psoriásica. La artritis reactiva, la espondilitis anquilosante juvenil y la artritis enteropática representaron el 2,5% cada una. La confiabilidad test-retest del BASDAI y del BASFI fueron ambas de 0,99 (p<0,0001). La consistencia interna para el BASDAI fue de 0,88 (p=0,002), coeficiente de correlación intraclase para consistencia interna: 0,9867 (p=0,001).
Consistencia interna para el BASFI: α de Cronbach: 0,7985 (p=0,002), coeficiente de correlación intraclase para consistencia interna: 0,9055 (p=0,001).
La validez de constructo del BASDAI fue de 0,84 para estado de bienestar general del paciente y 0,84 para el recuento de entesis (p<0,0001).
ConclusiónLa versión venezolana del BASDAI/BASFI podría utilizarse en la investigación clínica para evaluar el curso de la actividad de la enfermedad en pacientes venezolanos con EsA.
Spondyloarthropathies (SpA) are a group of diseases which have a serious impact on quality of life,1,2 and high indirect and tangible costs2–4 that have major implications for family life, social life and employment.4–6 During the course of the disease, axial and peripheral joint involvement lead to major disabilities with limitation of functional abilities.4,5,7
The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) is a questionnaire which may be self-administered and has been created to measure the disease activity in patients with ankylosing spondylitis (AS).7 The original version in English consists of 6 questions which assess 5 key components for understanding the disease, and these are: fatigue, back pain, join pain/inflammation, entheses pain and morning stiffness. This tool has demonstrated its reliability, validity and sensitivity to change and it is viably applicable to daily clinical practice.8,9
BASDAI has been used in a wide range of studies10–12 and forms part of the treatment response criteria for treatment with NSAIDS, disease-modifying antirheumatic drugs (DMARDS) and even with biologic therapies.13,14 The Assessment of SpondyloArthritis international Society group (ASAS) proposes it in their guides as a criteria for initiating anti-tumour necrosis factor therapies.15,16 In contrast the Bath Ankylosing Spondylitis Functional Index (BASFI) mainly assesses functional ability, health status and the evolution of the patient who presents with AS.17 Both questionnaires are complementary to one another and commonly used by all rheumatologists, validated into several languages (Italian, Portuguese, German, etc.), and Spanish versions also exist in Spain, Mexico and Argentina.18–24
The aim of this study was to evaluate the reliability, construct validity and feasibility of the BASDAI and BASFI in a Venezuelan version.
Patients and methodsThis study was conducted using a descriptive, cross-sectional design for validation. It included 81 patients out of a population of 2232 patients from an outpatient department of Rheumatology in the Hospital Universitario of Caracas and the Rheumatology Unit of Complejo Hospitalario Universitario Ruiz y Páez (CHURYP) in Ciudad Bolívar, Bolívar State, attended between January 1980 and December 2007. Inclusion criteria were: (1) to meet with the diagnostic criteria of Amor et al. for SpA or with the modified New York criteria for AS; (2) patients of either sex, with variability in clinical activity criteria according to the European Study of Spondyloarthropathies Group criteria (ESSG)14; (3) literacy: literate people which a minimum educational level up to 6th grade primary and (4) written consent from the patient to participate in the study. The exclusion criteria were: (1) suspicion of another autoimmune rheumatic, metabolic or infectious disease (concomitant), (2) not meeting with any of the requirements considered as inclusion criteria and (3) pregnancy. Of the 81 patients, only 40 were included in the pilot group for several reasons: lack of recording of address or telephone in the medical record, change of address or telephone number, residing a long way away, withdrawal from healthcare service unit, death, etc.
VariablesThe patients were selected after reviewing their clinical records and investigating whether they met with the inclusion criteria. They were then contacted by telephone and were given a date for evaluation. The following variables were recorded: age, sex, civil status, educational level, profession, whether employed or not, origin, duration of illness in years, current treatment (NSAIDS, corticoids, DMARDS, biologics), patient's general state of wellbeing during the previous week (GSW), enthesis count (ETCO), low back pain intensity (LBPI), duration of morning stiffness (DUMS), Schöber test (Schöber-t), distance between occiput and wall (OWD), chest expansions (CHEX) and erythrocyte sedimentation rate (ESR).
The patients selected for the study (N=40) were given the BASDAI and BASFI tools in their Spanish Venezuelan version, which was obtained by following the internationally accepted standardised norms for cross-cultural validation and adaptation of health measurement questionnaires.4 They were applied by a single medical interviewer to reduce the interobserver margin of error. Two visits were made: during the first, after obtaining written consent from the patient, demographic variables were recorded, in addition to those previously mentioned concerning the disease. The questionnaires were then given to the patient and the corresponding clinical assessment was made, after which a blood sample was taken to measure early hour ESR (using the Westergreen method). All samples were assessed by the same bio analyst, in the laboratory of the Rheumatology Unit, to avoid interobserver bias. In the second visit, 24h after the first one, the retest was carried out.
Cross-cultural adaptation and validation of the activity index of the disease and the functional index for ankylosing spondylitisCross-cultural adaptation included translation into standard language plus changes to words into the cultural and idiomatic context and changing items, whenever necessary, to match the same concept.25 The steps taken in validation were:
Translation and adaptationThe translation into Spanish of the original tools in English by 3 bilingual rheumatologists. A review of the tool translated by another 2 rheumatologists was made to evaluate semantics, language, conceptualization and obtainment of the final version in Venezuelan Spanish of the tools. The following changes were made: in item number
5 of the BASFI, “getting up without help when lying on one's back on the floor.” The term “lying on one's back on the floor.” was replaced by “facing upwards on the floor”. In item number 3 of the BASDAI, “how would you describe the degree of pain you have had in your neck, back or hips” the word “hip” was replaced by “waist”. A certified translator did the translation of the tools from Spanish (final version in Venezuelan Spanish) to English. The new translation of the questionnaires in English was compared with the original version so that the necessary changes could be made to the final Spanish version.
Reproducibility or trustworthinessThis was carried out through evaluation of the temporary stability of the tools (application of test and retest) and their own internal consistency. The questionnaires were distributed to 40 patients and again 24h later. The time used was measured (in seconds) as response to the tools on the 2 opportunities. The questions were answered on a visual analogue scale with a score between 1 and 10 (1=none; 10=very severe).
ValidationValidity of the tool construct was made comparing the score of each of the tools with the following clinical variables: ETCO, GSW of the patient during the last week, intensity of back pain, DUMS and ESR. The comparison of the BASFI with the evaluations of the spinal mobility was made with the modified Schöber-t, the OWD and the CHEX. Measurement was taken with arms by the side of the body.
Statistical analysisDescriptive statistics were used to describe the sample which included average, standard deviation (SD) and median for continuous variables. For categorical variables percentile distribution was used. The Pearson correlation coefficient was used to analyse reliability or reproducibility of the tools (test–retest), and for analysis of the tool construct validity. Cronbach's α coefficient and the intraclass correlation coefficient were used for assessing internal consistency. Statistical analysis was made using the SAS system for Windows version 9.1.3 (SAS Institute, Cary, NC)
ResultsThe self-administered questionnaires BASFI and BASDAI in their Venezuelan Spanish version were completed by 40 patients with diagnosis of SpA, whose demographics are contained in Table 1.
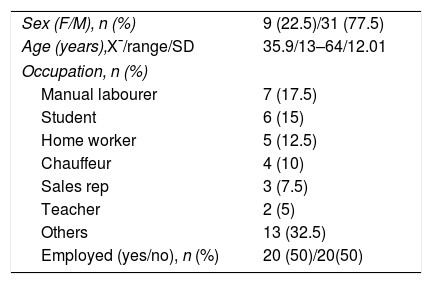
Demographic characteristics of patients diagnosed with spondyloartritis.
| Sex (F/M), n (%) | 9 (22.5)/31 (77.5) |
| Age (years),X¯/range/SD | 35.9/13–64/12.01 |
| Occupation, n (%) | |
| Manual labourer | 7 (17.5) |
| Student | 6 (15) |
| Home worker | 5 (12.5) |
| Chauffeur | 4 (10) |
| Sales rep | 3 (7.5) |
| Teacher | 2 (5) |
| Others | 13 (32.5) |
| Employed (yes/no), n (%) | 20 (50)/20(50) |
F: female; M: male.
The age range was from 13 to 64 years, with an average±SD of 35.9±12 years. The majority were men (77.5%). 17.5% were manual labourers, 15% students, 12.5% home workers, 10% chauffeurs, 7.5% sales representatives, 5% teachers and 32.5% diverse employment. 50% of the patients had permanent employment.
Regarding the level of education of the patients: the majority (37.5%) had completed secondary education, 35% had not completed secondary education, 20% were university graduates and 7.5% had a primary education level.
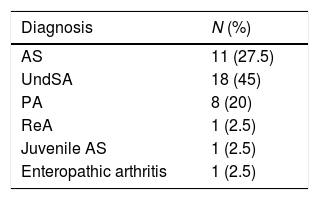
Table 2 shows the main diagnoses of the patients, which were: undifferentiated spondyloarthritis (45%), AS (27.5%) and psoriatic arthritis (20%).
Distribution of patients with spondyloartritis (SpA), n=40.
| Diagnosis | N (%) |
|---|---|
| AS | 11 (27.5) |
| UndSA | 18 (45) |
| PA | 8 (20) |
| ReA | 1 (2.5) |
| Juvenile AS | 1 (2.5) |
| Enteropathic arthritis | 1 (2.5) |
PA: psoriatic arthritis; ReA: reactive arthritis; AS: ankylosing spondylitis; UndSA: undifferentiated spondyloartritis; juvenile AS: juvenile spondylitis.
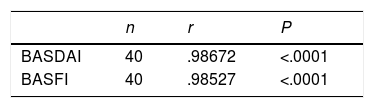
Table 3 contains the Pearson correlation coefficient values (r) for the retest of the Venezuelan Spanish version of the BASDAI and the BASFI. The correlation (r) between the 2 administrations of the tool was high, with a value of r: .98672, which was higher than the value considered ideal, which is r: .80; this result was statistically significant, P<.0001. The correlation between the 2 administrations of BASFI gives a value of r=.98527, which is considered high, with a value above the considered optimum of .8. The Student's paired test showed that the result was statistically significant (P<.0001).
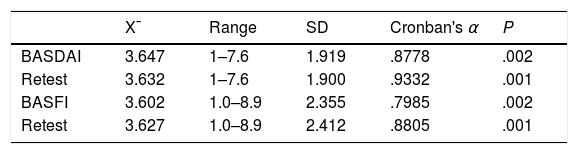
Table 4 shows the result of Cronbach's α reliability coefficient for the application of the BASDAI in Venezuelan Spanish. The tool has a high reliability value, since it is above the value considered ideal, which is r: .80, both in the first application and the second, with the optimum being the second application, with a result higher than the parameter shown, that was statistically significant (P=.001). Cronbach's α reliability coefficient for the application of BASFI in Venezuelan Spanish (Table 4) gave a high reliability level of the tool, being optimum in its second application with a result higher than that considered optimum (r: .8) and statistically significant (P=.001).
Cronbach's alpha coefficient of reliability from application of the BASDAI and BASFI in Venezuelan Spanish.
| X¯ | Range | SD | Cronban's α | P | |
|---|---|---|---|---|---|
| BASDAI | 3.647 | 1–7.6 | 1.919 | .8778 | .002 |
| Retest | 3.632 | 1–7.6 | 1.900 | .9332 | .001 |
| BASFI | 3.602 | 1.0–8.9 | 2.355 | .7985 | .002 |
| Retest | 3.627 | 1.0–8.9 | 2.412 | .8805 | .001 |
BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index Test; Retest: repetition of the same test on a second occasion.
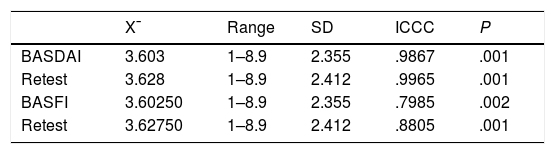
Table 5 gives the result of the intraclass correlation for the application of the BASDAI in Venezuelan Spanish. This measure of internal consistency reports that the test has a high internal consistency with results both in the first and second applications which are statistically significant (P=.001); considering a value above r: .80 as optimum, with the result being higher than this in both applications of the tool. Table 5 shows the result of the intraclass correlation for the application of the BASFI in Venezuelan Spanish. A high level of reliability was shown in the two tool applications, with the ideal value as r: .80 and the result more optimum in the second application, with a value higher than that shown and statistically significant (P=.001).
Intraclass correlation coefficient for the BASDAI in Venezuelan Spanish.
| X¯ | Range | SD | ICCC | P | |
|---|---|---|---|---|---|
| BASDAI | 3.603 | 1–8.9 | 2.355 | .9867 | .001 |
| Retest | 3.628 | 1–8.9 | 2.412 | .9965 | .001 |
| BASFI | 3.60250 | 1–8.9 | 2.355 | .7985 | .002 |
| Retest | 3.62750 | 1–8.9 | 2.412 | .8805 | .001 |
BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASFI: Bath Ankylosing Spondylitis Functional Index Test; Retest: repetition of the same test on a second occasion.
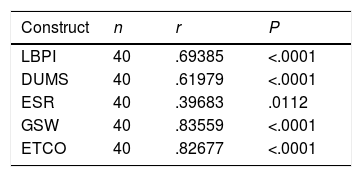
Table 6 shows the Pearson correlation coefficient values (r) obtained for the selected domains as indicative of the disease activity (validity of construct) and the BASDAI. The highest correlation was obtained for GSW with an r of .83559, followed by ETCO, r: .82677 (P<.0001), being statistically significant.
Construct validity of the in Venezuelan Spanish version of the BASDAI. Pearson's correlation coefficient (r).
| Construct | n | r | P |
|---|---|---|---|
| LBPI | 40 | .69385 | <.0001 |
| DUMS | 40 | .61979 | <.0001 |
| ESR | 40 | .39683 | .0112 |
| GSW | 40 | .83559 | <.0001 |
| ETCO | 40 | .82677 | <.0001 |
ETCO: enthesis count; GSW: general state of wellbeing of the patient during the last week; DUMS: duration of morning stiffness; LBPI: low back pain intensity; ESR: erythrocyte sedimentation rate.
A lower but statistically significant correlation was obtained for LBPI, r: .69385, and for DUMS, r: .61979 (P<.0001). The correlation between ESR and BASDAI was low, r: .39683 (P=.0112). The results of this research refer to the mean of stability or test–retest after 24h of the Venezuelan Spanish version of the BASDAI allowing a high level of reliability to be demonstrated, with a Pearson correlation coefficient (r: .98672, P<.0001), showing the consistency in the 2 applications of the tools, and being statistically significant (P<.001).5,22
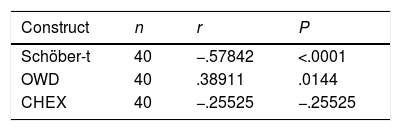
Table 7 shows the Pearson correlation coefficient values (r) between the selected constructs to assess functionalism and BASFI. A very low correlation was obtained between OWD and BASFI, r: .38911 (P=.014), which was statistically significant. There was no correlation between the Schöber-t, the CHEX and the BASFI.
The BASDAI in Venezuelan Spanish had a high internal consistency (Table 7), since it presented a Cronbach α coefficient which was considered above the ideal value of r: .8 (first application: .8778, second application: .9332), and was statistically significant (P=.001). This result was above that obtained by the Spanish version of BASDAI, whose internal consistency showed a Cronbach α coefficient of r .870 (P<.00001). This demonstrated high reliability of our index. The BASFI in Venezuelan Spanish also gave a high internal consistency, with a Cronbach α coefficient of .7985(P=.002) in its first application and r: .8805 (P=.001) in the second application.
DiscussionThe group of diseases comprising SpA are chronic, highly incapacitating conditions with a severe impact on quality of life, generating repercussion for family life, social life and employment.26 They are characterised by low back pain, stiffness, limitation of movement in the spine, fatigue and depression which leads to a loss of functional capacity. The definition of the disease activity is complex and not fully established. In the Outcome Measures in Rheumatology Clinical Trials (OMERACT-IV) congress, celebrated in 1998, the ASAS group created a selection of clinimetric domains and instruments for assessing the course of the disease in clinical practice.27,28
The selected domains were: overall evaluation of patient status, low back pain, back stiffness, physical function, affected joints, enthesitis, fatigue, acute phase reactants and conventional radiology of the spine which includes the sacroiliac joints. To assess the disease activity the BASDAI was selected and to assess physical function the Bath (BASFI) functional index or the Dougados Functional Index (DFI) was selected.5–8,29
The BASDAI questionnaire includes the main components encompassing the concept of activity, assessed from the patient's viewpoint. Although it does not include “hard” data, such as acute phase reactants, or the viewpoint of the physician, and could be considered overly subjective, there are several facts which support its use as a tool for measuring AS activity and it therefore could be considered to meet all the conditions required by the OMERACT-IV for measuring tools in the sense of being genuine (valid), having discriminatory capacity (reliability, and sensitivity to change) and feasibility.26,29
The BASDAI has demonstrated excellent correlation with acute phase reactants.8,26,27 In this study the validation of these 2 cross-culturally adapted tools into Venezuelan Spanish was carried out in keeping with internationally accepted standardised for providing cross-cultural validation and adaptation to health measurements proposed by Guillemin et al.,25 as had occurred in previous studies and the tools were analysed in terms of trustworthiness (reproducibility and internal consistency) and validity of constructs.
Regarding the reproducibility of the BASFI version obtained in this study, a high level of reliability was shown in reference to the measurement of stability or test–retest after 24h, with a Pearson correlation coefficient (r: .98527, P<.0001), the result of which was higher than the original English version and the Mexican version: r: .89, P<.0001 and r: .68, respectively. The latter was expressed as an intraclass correlation, with a 95% confidence interval of .29–.85.8,22
Validity of construct refers to the degree in which a tool measures the variable it is studying. In the BASDAI version in Venezuelan Spanish, on correlating the results of the clinimetrically administered tools and the constructs related to disease activity: low back pain, DUMS, early hour ESR, GSW and ETCO, the highest correlation was for GSW of the patient during the last week (r: .83559), followed by ETCO (r: .82677).
The difference between the correlations of these constructs and the BASDAI was statistically significant, P<.0001. According to that established in this study, the significance of r as higher or equal to .8, was high. A lower correlation was obtained by low back pain (r: .69385), followed by DUMS (r: .61979), with a P<.0001. The ESR obtained the lowest correlation on correcting it with the disease activity (r: .39683, P=.0112).
The ASAS group demonstrated that measuring of the patient's GSW during the previous week is a major measurement in clinical practice which, although simple, is highly trustworthy in patient evaluation.30 Although it is a subjective parameter and dependent upon an emotional state and how the patient feels when the assessment is made,30,31 it was corroborated that a relationship does exist with the level of disease activity.
In the main domains of the ASAS (core sets) group inflammatory low back pain was regarded as the main symptom of AS; this symptom is the most useful indication for monitoring both activity and progression of the disease, with a sensitivity and specificity of 75% for the diagnosis of axial disease.26,30 However, in our study, we found that the correlation between low back pain and the BASDAI was low, r: .69385. We believe that this may be due to the fact that the number of patients included with different types of SpA was not balanced, with AS having a lower number (27.5%) of patients. This lessened the importance of the symptom which was not statistically significant, with the main symptom in this entity being inflammatory type pain, unlike the other entities comprising SpA, where there are more diverse clinical manifestations, even predominantly peripheral.10,17
The use of acute phase reactants and particularly ESR was a point of controversy. Some authors found a good correlation between the ESR and the BASDAI,32,33 whilst others differed and highlighted that the ESR is not a reliable activity marker, since in up to 40% of patients with active AS there was no raising of the acute phase reactants despite activity taking place and that generally they were raised in patients with peripheral joint compromise or who had extra-joint involvement, such as intestinal inflammatory disease.34,35 In our study, the ESR did not show a good correlation with disease activity (r: .39683, P=.0112).
Idiomatic adaptations or linguistic changes to a simple word such as sock (“calcetín” in Castilian Spanish) which is not commonly used in Venezuela and was replaced by the word tights (“media” or “panty” in the case of women),36 changed this question in the BASMI to interpret what we really wished to ask the patient. The population mentioned that this morpheme was translated without considering the cultural influence or adaption into the other language, because of the cultural influence of the speakers. As in item 4 of BASDAI, where we want to explore "enthesitis" and replace the question with "sites sensitive to touch or pressure".
Even the operating systems (Windows®) (Microsoft®, version 3.0, New Mexico, USA) currently contain in their Word processors (like Word®) Spanish from Mexico, Spanish from Puerto Rico, and from other countries where Spanish is spoken,37 and replace an adjective, a noun or a verb into the idiomatic expression of the country where the user resides, since word sequences would often not be understood or worse still, would confuse the reader. In this study our patients understood the 5th question better from the Venezuelan Spanish version of the BASFI: “Getting up without help lying facing upwards on the floor” than the version translated literally from the English: “Lying on one's back on the floor and getting up without help.”36
Etymologically speaking, the notion of the verb “lean forward” (“inclinarse” in Spanish), instead of “bend forward” (“doblarse” in Spanish) to pick up a pencil from the floor without help (in reference to item 2 of the BASFI) is neither an idiom nor a regional dialect; this question has a level of complexity which in the Spanish version should be between brackets “bending forward at the waist” (“doblando la cintura”). It is important to reiterate that in our sample the majority of subjects had completed secondary education (>50%), and 20% had a university degree.
To conclude, with this study we have produced a Venezuelan Spanish version of BASDAI which is valid and reproducible as a clinical tool of measurement. It is reliable for assessing the disease activity in patients suffering from SpA and the same may be said for the Venezuelan version of the BASFI with regard to its reproducibility (supplementary material) contains the version we had been using since 2004 in our clinics, which was the Spanish questionnaire recommended by the Spanish Society of Rheumatology (Spanish acronym SER), extensively used by the Spanish speaking countries where their own questionnaires had not yet been validated.38
Ethical disclosuresProtection of people and animalsThe authors declare that no experiments were carried out on humans or animals for this research.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors obtained the informed consent from the patients and/or subjects referred to in this article. This document is held by the corresponding author.
Conflict of interestsThe authors have no conflict of interests to declare. They did not receive any fees from any public or private entity.
Please cite this article as: Rauseo Vera M, Gutiérrez-González LA, Maldonado I, Al Snih S. Validación al idioma español de Venezuela de los índices de actividad de enfermedad y el índice funcional para espondilitis anquilosante. Reumatol Clin. 2019;15:223–228.