Subglottic stenosis (SGS) in granulomatosis with polyangiitis (GPA) may result from active disease or from chronic recurrent inflammation. The objective of the study was to describe the clinical features and treatment of patients with subglottic stenosis.
MethodsWe retrospectively reviewed the medical records of all patients with SGS due to GPA diagnosed at Rheumatology department between January 2000 and June 2015.
ResultsWe present 4 cases of SGS at our department during a period of 15 years. The interval between the presentation of the GPA and SGS varied between 2 and 144 months. The leading symptoms of SGS were dyspnea on exertion and stridor. Three patients presented SGS without evidence of systemic activity. Two patients presented SGS grade i and received tracheal dilatation; two recurred and three needed a tracheostomy due to severe airway-limiting stenosis.
ConclusionSGS presents high morbidity. Even though subglottic dilatation provides symptomatic relief, recurrences may present. Severe airway-limiting stenosis often requires tracheostomy.
La estenosis subglótica (ESG) en la granulomatosis con poliangitis (GPA) puede ser consecuencia de la enfermedad activa o de procesos inflamatorios repetitivos. Nuestro objetivo es describir las características clínicas y el tratamiento de los pacientes con ESG.
MétodosEstudio descriptivo retrospectivo de los casos diagnosticados durante el período comprendido entre el 1 de enero del 2000 y el 1 de junio del 2015.
ResultadosPresentamos 4 casos; la ESG se presentó entre los 2 y 144 meses del diagnóstico de la GPA, los síntomas de presentación fueron disnea de esfuerzo y el estridor laríngeo, 3 desarrollaron ESG en ausencia de actividad sistémica. Dos sujetos con ESG grado i fueron tratados con dilatación traqueal, 2 casos presentaron reestenosis y en 3 casos fue necesario la realización de traqueostomía.
ConclusiónLa ESG presenta una alta morbilidad. La dilatación endoscópica proporciona alivio sintomático; sin embargo, suelen existir recidivas de la estenosis. La obstrucción grave de la vía aérea a menudo requiere de traqueostomía.
Granulomatosis with polyangiitis (GPA) is a systemic autoimmune disease of unknown etiology. It is characterized by necrotizing granulomatous inflammation of the respiratory tract and vasculitis that affects small- and medium-sized blood vessels.1 Stenosis of subglottis and proximal trachea (SGS) can result from active disease or from recurrent inflammatory processes. An incidence of 8%–23% has been reported during the course of GPA, and it can be the first manifestation in 1%–6% of the patients.1–6 The objective of this article is to describe the clinical characteristics and treatment of patients with SGS.
Materials and MethodsWe conducted a retrospective descriptive study of the cases diagnosed during the period between 1 January 2000 and 1 June 2015 in the Centro Médico Nacional Siglo XXI, a tertiary care referral center in Distrito Federal, Mexico. In order to compare our findings with the results of other published series, we performed a search of the available medical literature by means of a systematic review in the PubMed and EMBASE databases. It was limited to articles published in English or Spanish, using the following search terms: “subglottic stenosis”, “tracheal stenosis”, “Wegener's granulomatosis” and “granulomatosis with polyangiitis”. We included those studies that provided demographic data, the level of disease activity, the description of the therapy employed and the outcomes. Case reports were excluded.
ResultsWe present 4 cases of SGS in patients, predominantly women, with GPA; the presenting symptoms were exertional dyspnea and laryngeal stridor in every case; SGS was diagnosed between 2 and 144 months after the onset of GPA; 3 patients developed SGS in the absence of systemic activity; all the patients had chronic sinusitis. Three individuals had saddle nose, and 1 showed no evidence of antineutrophil cytoplasmic antibodies (Table 1). Two patients had grade I SGS and were treated with glucocorticoids and tracheal dilatation; restenosis developed in 2 patients, and 3 required tracheostomy due to severe airway compromise (grades III and IV SGS according to the Myer-Cotton grading system). During follow-up, decannulation was possible in 1 patient.
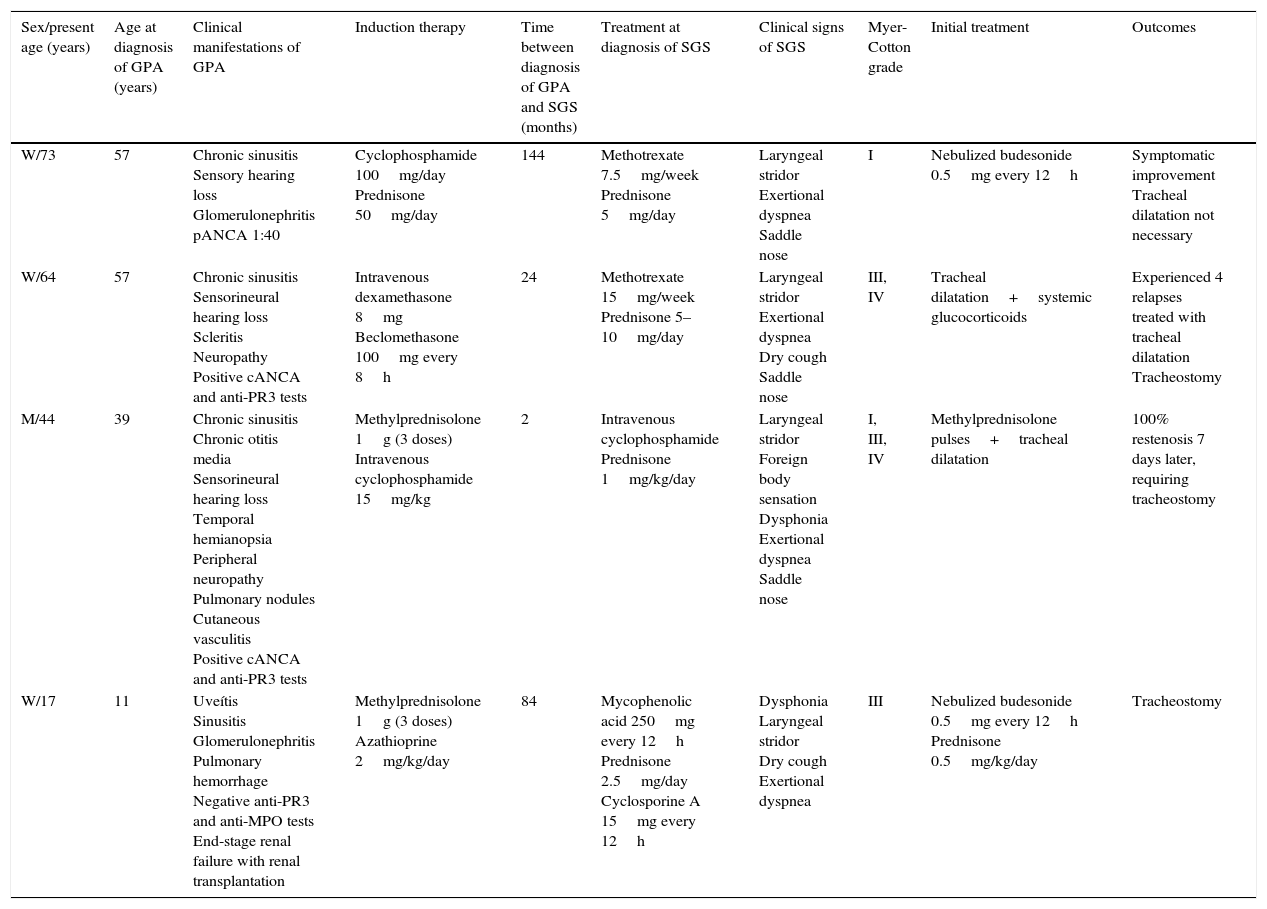
Clinical Characteristics of Patients With Granulomatosis With Polyangiitis and Subglottic Stenosis.
| Sex/present age (years) | Age at diagnosis of GPA (years) | Clinical manifestations of GPA | Induction therapy | Time between diagnosis of GPA and SGS (months) | Treatment at diagnosis of SGS | Clinical signs of SGS | Myer-Cotton grade | Initial treatment | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| W/73 | 57 | Chronic sinusitis Sensory hearing loss Glomerulonephritis pANCA 1:40 | Cyclophosphamide 100mg/day Prednisone 50mg/day | 144 | Methotrexate 7.5mg/week Prednisone 5mg/day | Laryngeal stridor Exertional dyspnea Saddle nose | I | Nebulized budesonide 0.5mg every 12h | Symptomatic improvement Tracheal dilatation not necessary |
| W/64 | 57 | Chronic sinusitis Sensorineural hearing loss Scleritis Neuropathy Positive cANCA and anti-PR3 tests | Intravenous dexamethasone 8mg Beclomethasone 100mg every 8h | 24 | Methotrexate 15mg/week Prednisone 5–10mg/day | Laryngeal stridor Exertional dyspnea Dry cough Saddle nose | III, IV | Tracheal dilatation+systemic glucocorticoids | Experienced 4 relapses treated with tracheal dilatation Tracheostomy |
| M/44 | 39 | Chronic sinusitis Chronic otitis media Sensorineural hearing loss Temporal hemianopsia Peripheral neuropathy Pulmonary nodules Cutaneous vasculitis Positive cANCA and anti-PR3 tests | Methylprednisolone 1g (3 doses) Intravenous cyclophosphamide 15mg/kg | 2 | Intravenous cyclophosphamide Prednisone 1mg/kg/day | Laryngeal stridor Foreign body sensation Dysphonia Exertional dyspnea Saddle nose | I, III, IV | Methylprednisolone pulses+tracheal dilatation | 100% restenosis 7 days later, requiring tracheostomy |
| W/17 | 11 | Uveítis Sinusitis Glomerulonephritis Pulmonary hemorrhage Negative anti-PR3 and anti-MPO tests End-stage renal failure with renal transplantation | Methylprednisolone 1g (3 doses) Azathioprine 2mg/kg/day | 84 | Mycophenolic acid 250mg every 12h Prednisone 2.5mg/day Cyclosporine A 15mg every 12h | Dysphonia Laryngeal stridor Dry cough Exertional dyspnea | III | Nebulized budesonide 0.5mg every 12h Prednisone 0.5mg/kg/day | Tracheostomy |
ANCA, antineutrophil cytoplasmic antibodies; anti-MPO, anti-myeloperoxidase antibodies; anti-PR3, anti-proteinase 3 antibodies; GPA, granulomatosis with polyangiitis M, man; SGS, subglottic stenosis; W, woman.
A 73-year-old woman who had been diagnosed with GPA at the age of 57 years was being treated with methotrexate at 15mg/week and prednisone at 5mg/day. She presented with dry cough, laryngeal stridor and dyspnea. Laryngoscopy revealed SGS of 50%; she had a score of 6 on the Birmingham Vasculitis Activity Score (BVAS). She was treated with 8mg intravenous dexamethasone and 0.5mg nebulized budesonide every 12h, and her symptoms improved with no additional procedures. Six months later, laryngoscopy revealed SGS of 30% and no further treatment was required.
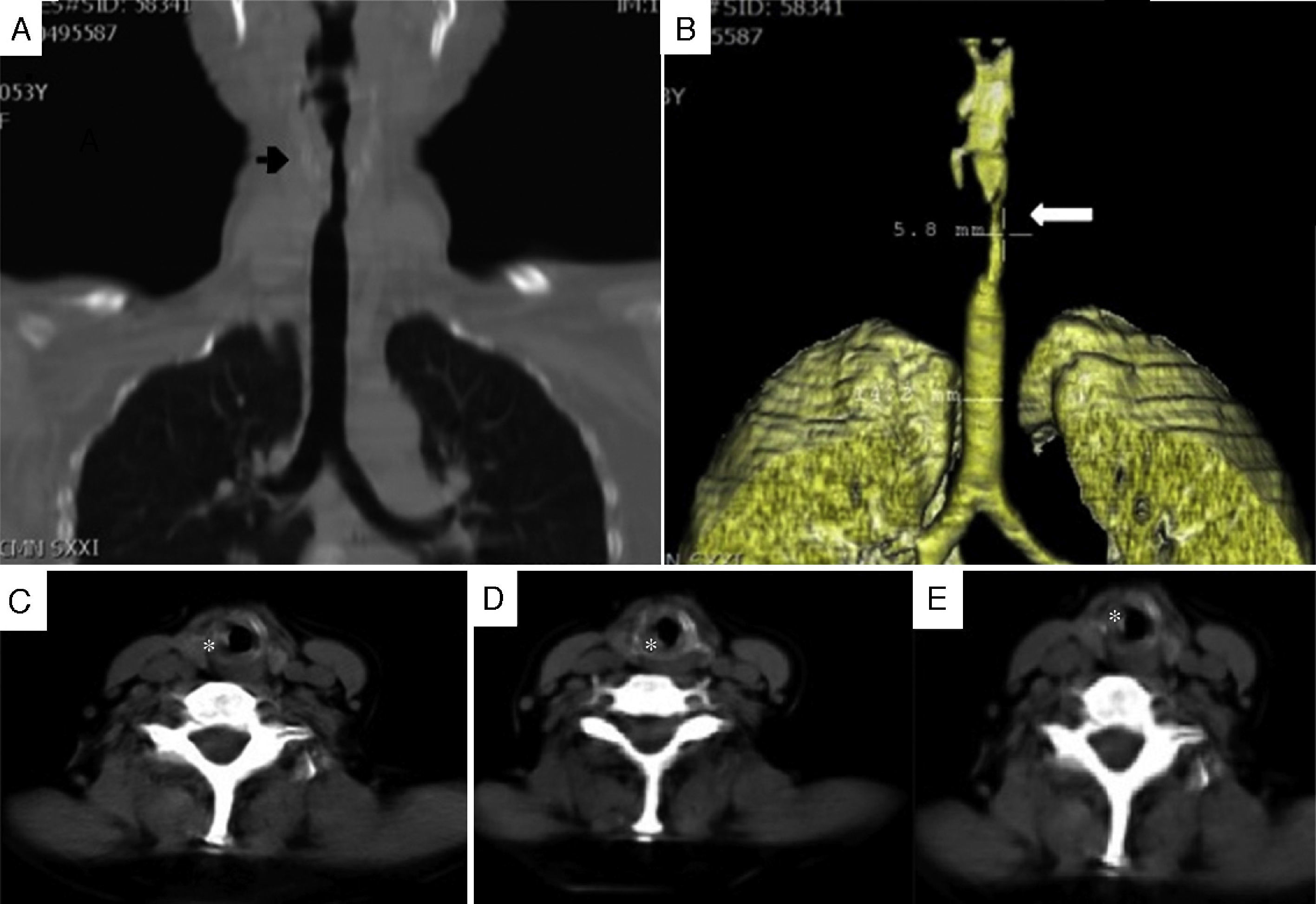
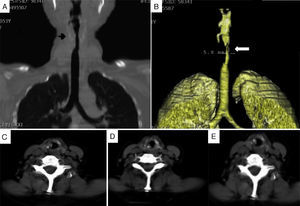
Patient no. 2A 64-year-old woman who had been diagnosed with GPA at the age of 57 years. At the age of 59, she presented with dyspnea and stridor. Computed tomography (CT) of larynx and trachea revealed a granulomatous lesion obstructing the tracheal lumen (Fig. 1). Bronchoscopy disclosed an obstruction of 75% in subglottis, and tracheal dilatation and tracheostomy were performed. Two months later, the patient presented with restenosis of 85% requiring tracheal dilatation, and 2 weeks after that, SGS of 50% was detected, and tracheoplasty was performed using the technique of Grillo and Pearson. The patient required repeat dilatation 18 months later due to SGS of 80%. Fifteen months after that last dilatation, bronchoscopy revealed SGS of 30%, and resolution of dyspnea and stridor. As restenosis did not occur over the following 7 months, decannulation was possible.
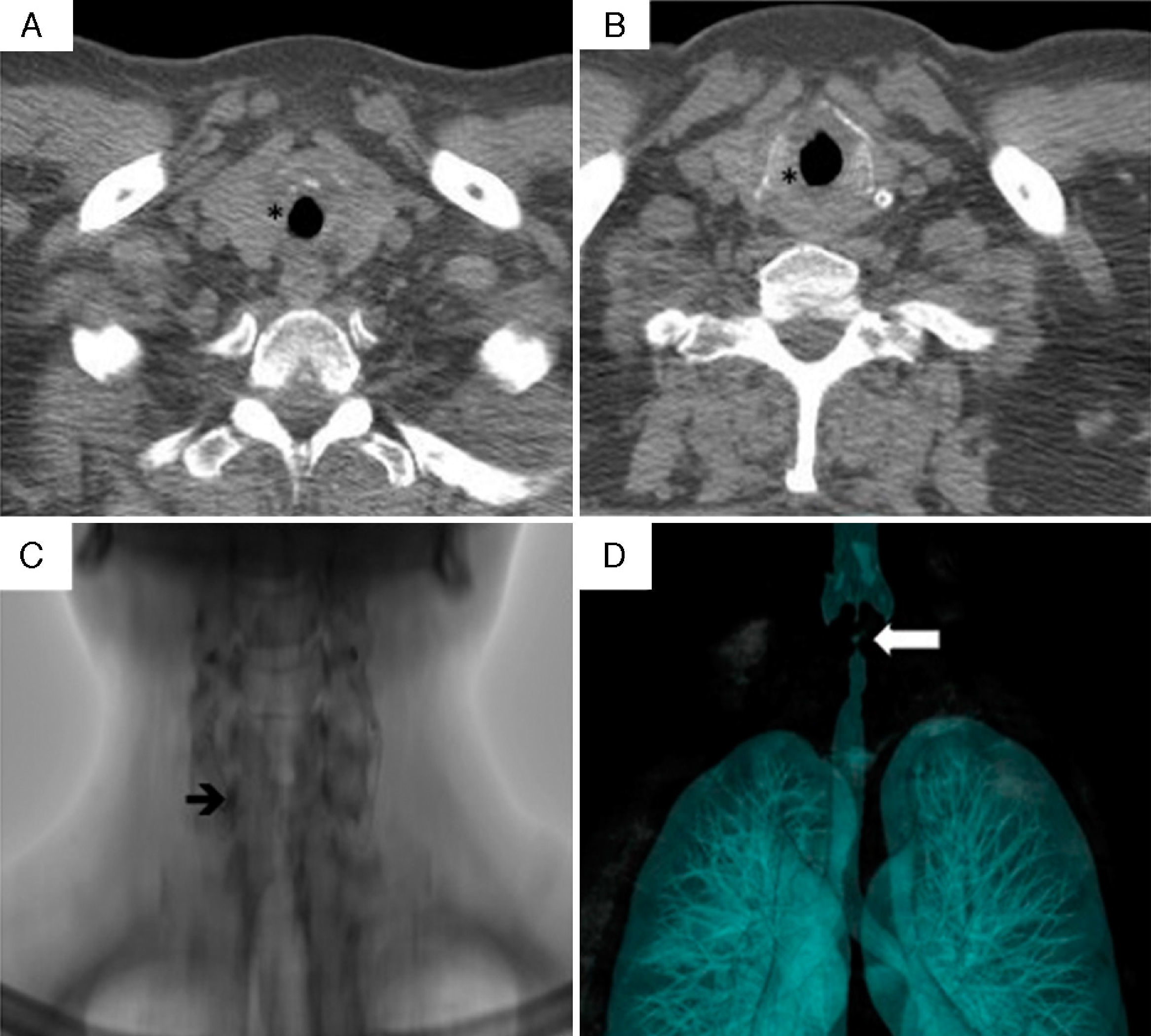
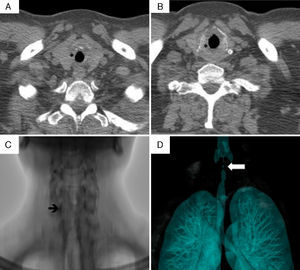
Patient no. 3A 44-year-old man had been diagnosed with GPA at the age of 39 years. Two months after the diagnosis, he presented with laryngeal stridor, dyspnea, foreign body sensation in his larynx and dysphonia. Fiberoptic laryngoscopy revealed SGS of 20% with systemic activity (BVAS score of 23). He was treated with 3 doses of 1g/day of methylprednisolone and intravenous cyclophosphamide; the systemic manifestations remitted, but his dysphonia, stridor and dyspnea progressed. One month later, CT revealed concentric circumferential thickening that partially obstructed the tracheal lumen (Fig. 2 A and B). Bronchoscopy showed SGS of 70%, with erythematous laryngeal structures. He received 3 doses of 1g/day of methylprednisolone and underwent tracheal dilatation. Subsequently, the clinical course was satisfactory, with remission of the stridor and improvement in the symptoms. However, 7 days later, his symptoms recurred, with 100% restenosis (Fig. 2C and D), which required emergency tracheostomy.
Computed tomography of larynx and trachea in patient no. 3. (A and B) Axial images showing the subglottic stenosis with concentric circumferential thickening of the mucosa (*) partially obstructing the tracheal lumen. (C) Subglottic stenosis (arrow). (D) Volumetric reconstruction of subglottic stenosis (arrow).
A 17-year-old woman had been diagnosed as having GPA at the age of 11 on the basis of pulmonary and renal involvement. Eighty-four months after the diagnosis of GPA, she presented with a 2-month history of dysphonia, laryngeal stridor, dry cough and dyspnea, and a BVAS score of 3. Computed tomography revealed tracheal stenosis measuring 6cm in length, and a transverse diameter at the narrowest portion of 5mm. Tracheostomy was performed due to airway compromise and, after 2 months of follow-up, decannulation remained impossible.
The literature search yielded 77 studies, 12 of which evaluated the treatment of SGS in patients with GPA and met the selection criteria. As the study published by Langford et al.5 included the patients described in the study of Lebovics et al.,7 they were grouped in a single study. Table 2 summarizes the major findings of these studies.
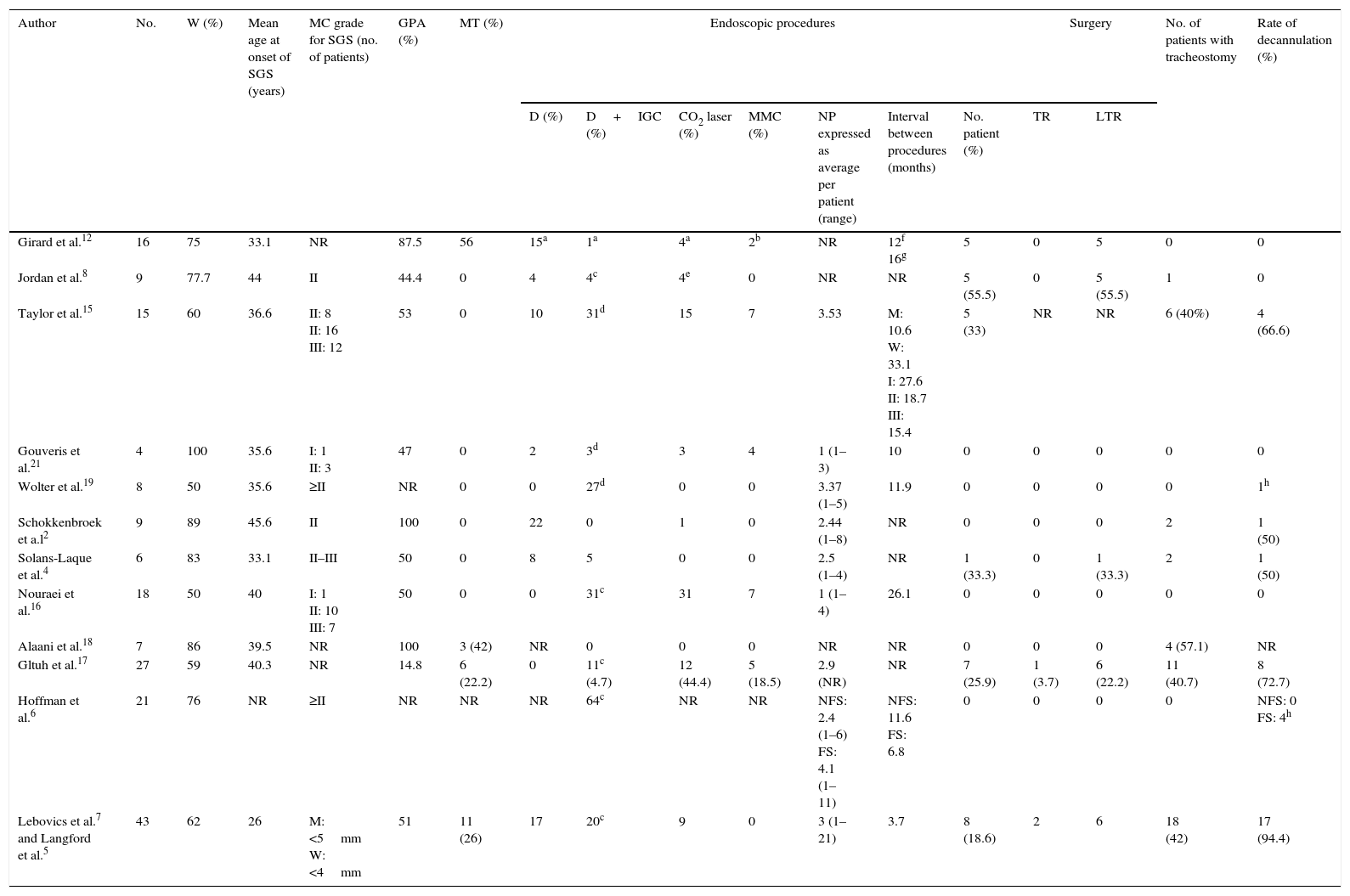
Summary of the Studies That Evaluate the Therapeutic Options in Subglottic Stenosis Associated With Granulomatosis With Polyangiitis.
| Author | No. | W (%) | Mean age at onset of SGS (years) | MC grade for SGS (no. of patients) | GPA (%) | MT (%) | Endoscopic procedures | Surgery | No. of patients with tracheostomy | Rate of decannulation (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D (%) | D+IGC (%) | CO2 laser (%) | MMC (%) | NP expressed as average per patient (range) | Interval between procedures (months) | No. patient (%) | TR | LTR | |||||||||
| Girard et al.12 | 16 | 75 | 33.1 | NR | 87.5 | 56 | 15a | 1a | 4a | 2b | NR | 12f 16g | 5 | 0 | 5 | 0 | 0 |
| Jordan et al.8 | 9 | 77.7 | 44 | II | 44.4 | 0 | 4 | 4c | 4e | 0 | NR | NR | 5 (55.5) | 0 | 5 (55.5) | 1 | 0 |
| Taylor et al.15 | 15 | 60 | 36.6 | II: 8 II: 16 III: 12 | 53 | 0 | 10 | 31d | 15 | 7 | 3.53 | M: 10.6 W: 33.1 I: 27.6 II: 18.7 III: 15.4 | 5 (33) | NR | NR | 6 (40%) | 4 (66.6) |
| Gouveris et al.21 | 4 | 100 | 35.6 | I: 1 II: 3 | 47 | 0 | 2 | 3d | 3 | 4 | 1 (1–3) | 10 | 0 | 0 | 0 | 0 | 0 |
| Wolter et al.19 | 8 | 50 | 35.6 | ≥II | NR | 0 | 0 | 27d | 0 | 0 | 3.37 (1–5) | 11.9 | 0 | 0 | 0 | 0 | 1h |
| Schokkenbroek et a.l2 | 9 | 89 | 45.6 | II | 100 | 0 | 22 | 0 | 1 | 0 | 2.44 (1–8) | NR | 0 | 0 | 0 | 2 | 1 (50) |
| Solans-Laque et al.4 | 6 | 83 | 33.1 | II–III | 50 | 0 | 8 | 5 | 0 | 0 | 2.5 (1–4) | NR | 1 (33.3) | 0 | 1 (33.3) | 2 | 1 (50) |
| Nouraei et al.16 | 18 | 50 | 40 | I: 1 II: 10 III: 7 | 50 | 0 | 0 | 31c | 31 | 7 | 1 (1–4) | 26.1 | 0 | 0 | 0 | 0 | 0 |
| Alaani et al.18 | 7 | 86 | 39.5 | NR | 100 | 3 (42) | NR | 0 | 0 | 0 | NR | NR | 0 | 0 | 0 | 4 (57.1) | NR |
| Gltuh et al.17 | 27 | 59 | 40.3 | NR | 14.8 | 6 (22.2) | 0 | 11c (4.7) | 12 (44.4) | 5 (18.5) | 2.9 (NR) | NR | 7 (25.9) | 1 (3.7) | 6 (22.2) | 11 (40.7) | 8 (72.7) |
| Hoffman et al.6 | 21 | 76 | NR | ≥II | NR | NR | NR | 64c | NR | NR | NFS: 2.4 (1–6) FS: 4.1 (1–11) | NFS: 11.6 FS: 6.8 | 0 | 0 | 0 | 0 | NFS: 0 FS: 4h |
| Lebovics et al.7 and Langford et al.5 | 43 | 62 | 26 | M: <5mm W: <4mm | 51 | 11 (26) | 17 | 20c | 9 | 0 | 3 (1–21) | 3.7 | 8 (18.6) | 2 | 6 | 18 (42) | 17 (94.4) |
D, mechanical dilatation; FS, stenosis with fibrotic scarring; GPA, granulomatosis with polyangiitis with systemic activity; IGC, intralesional glucocorticoid injection; LTR, laryngotracheal reconstruction; M, men; MC, Myer-Cotton grading system; MMC, mitomycin C; MT, medical treatment alone; NFS, stenosis with no fibrotic scarring; No., number of patients included in the study; NP, number of procedures; NR, not reported; SGS, subglottic stenosis; TR, tracheal resection; W, percentage of the subglottic stenosis patients who were women.
Subglottic stenosis occurs more frequently in women and young individuals (Table 2).4,7–12 The incidence of SGS was higher among patients with GPA onset during childhood or adolescence rather than during adulthood (48% vs 10%, P<.001).7 The subglottic region of the trachea is particularly susceptible to narrowing because of its small diameter, its lack of distensibility, the fragility of the tissue that lines it and its poor vascularization, which, if damaged, heals concentrically, further reducing the lumen.13
Subglottic stenosis is the result of inflammation, edema and fibrosis that typically extends from 3 to 4cm below the vocal chords.13 The active phase of GPA is accompanied by involvement of the tracheal mucosa, occasionally in the form of ulcers. As the vocal chords are seldom affected, in most cases the symptoms are mild or nonexistent. If the subclinical tracheal inflammation is not treated, fibrotic scar tissue forms within a variable period of time (the average is reported to be 39–60 months); when the lumen is already significantly compromised, dyspnea, cough, voice changes and stridor appear.2,10,14 Patients with SGS associated with GPA have been reported to have a higher incidence of paranasal sinus involvement and saddle nose (47% vs 23%, P<.01), and a lower rate of renal and pulmonary involvement compared to individuals with GPA without SGS.5,12 The most common complication of SGS is acute respiratory failure, which can be fatal. A case of a death due to acute respiratory failure caused by SGS has been reported.12
Early diagnosis depends entirely on a high degree of suspicion, as the symptoms are nonspecific until stridor appears. Laryngoscopy and CT with 3D reconstruction of the airway and virtual bronchoscopy are noninvasive techniques that make it possible to determine the extent and degree of stenosis. Bronchoscopy enables us to estimate laryngeal function, observe the characteristics of the lesion (eccentric or concentric, tracheal ulceration and inflammatory pseudopolyps), evaluate the extension and, eventually treat the changes in the airway.9,10
The presentation of SGS may be independent of the systemic manifestations of GPA and its treatment, and does not always follow the same course or respond to the treatment of the other organs affected by the vasculitis. Solans-Laqué et al. identified 2 groups based on the mode of presentation: (1) patients with isolated SGS; and (2) patients with systemic involvement and ear and upper airway manifestations that developed between 36 and 168 months after the diagnosis.4
The treatment will depend on the degree of obstruction and whether there is evidence of systemic activity; the objective is to reestablish the patency of the airway. Mild stenoses or reversible lesions can be treated with immunosuppressive agents and do not require surgical intervention. Tracheal dilatation is the most frequently utilized therapeutic procedure for the treatment of SGS; resection with CO2 laser and/or intralesional glucocorticoid injection are common adjuvants to dilatation.15 In general terms, it is recommended that manipulation of the airway be avoided during acute GPA activity, with the exception of life-threatening lesions, which should be treated by tracheostomy or stent placement.4,16,17 However, Nouraei et al.16 consider that the stenosis is a localized condition, and that the acute obstructive component can be treated with minimally invasive surgery, thus reducing the need for tracheostomy from 50% to 0 in patients with SGS secondary to inflammation of the mucosa in the absence of fibrotic scar tissue. Treatment exclusively with immunosuppressive agents in patients with systemic activity is controversial, as SGS does not always respond to systemic therapy, the rate of response ranging from 0 to 42%,5,12,17,18 and from 60% to 100% of the patients may require interventional strategies.2,10,12,15,16,19 Moreover, SGS can present while a patient is receiving immunosuppressive therapy for other manifestations of the disease.4–6,8,11,13,20 In some cases, the stenosis is so severe that temporary or permanent tracheostomy is required.4,13,58 Decannulation of the tracheostomy in GPA patients is difficult without the performance of additional procedures for the reconstruction of the airway.19 Endoscopic techniques like resection with CO2 laser have been utilized to remove the stenosis; however, the vasculitis and inflammation may result in extensive scar formation after the resection.5,19 Laryngotracheoplasty was useful in those patients with SGS in whom endoscopic procedures failed.17 Closure of the tracheostomy should be evaluated on an individual basis, and should be delayed until there is evidence that the airway is stable.7
If the patient has isolated SGS or when the obstruction is caused by fibrotic scar tissue in the absence of systemic activity, the administration of immunosuppressive therapy is not necessary. Management can consist of minimally invasive surgery, tracheal stent placement and, occasionally, end-to-end anastomosis.4,5
Restenosis can occur in approximately half of the patients (50%–75%) when GPA is in remission.12 Patients with local recurrence are generally treated with immunosuppressive therapy and repeated dilatations,4,5,16 with an average number of dilatations per patient of between 116,21 and 45,6 (range, 1–21). Patients treated with tracheal dilatation and intralesional glucocorticoid injections usually require dilatations every 12 to 18 months (range, 1 week to 26 months).4,5,11,12,22 The presence of fibrotic scar tissue influences the number of procedures and the interval between them; patients without fibrosis required an average of 2.4 procedures (interval, 11.6 months), whereas those with fibrosis required an average of 4.1 procedures (interval, 6.8 months).6 Better results have been reported in those individuals in whom intralesional glucocorticoid injection plus dilatation was performed prior to other types of surgery, especially laser therapy,6,19 although in another series, the use of laser or surgical resection was not associated with a higher risk of restenosis.12
It is difficult to establish the best therapeutic option because of the numerous strategies employed (both medical and surgical); moreover, they were frequently used in combination. On the basis of the results presented in Table 2, intralesional glucocorticoid injection with mechanical dilatation could be considered the therapy of choice. This procedure has been found to produce favorable results: it reduces the numbers of tracheostomies and the need for surgery, and enables decannulation in patients who have undergone tracheostomy. Its safety profile is good, although complications, such as tongue laceration (1 in 36 procedures19) and pneumothorax (3 in 177 procedures5,6) have been reported. However, this therapeutic approach was not available for our patients and, thus, we have no experience with its use in our population.
ConclusionSubglottic stenosis in GPA is associated with a high rate of morbidity. The combination of glucocorticoids and immunosuppressive agents is useful in the systemic disease, but not in SGS, since the latter can develop during the treatment. The management of SGS in GPA is complex and requires a multidisciplinary team. Endoscopic procedures alleviate the symptoms; however, the stenosis usually recurs in the majority of the patients. Severe airway obstruction often requires tracheostomy.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis manuscript has received no governmental or commercial financial support.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Horta-Baas G, Hernández-Cabrera MF, Catana R, Pérez-Cristóbal M, Barile-Fabris LA. Estenosis subglótica en granulomatosis con poliangitis (granulomatosis de Wegener): presentación de 4 casos. Reumatol Clin. 2016;12:267–273.