To systematically assess the literature related to the occurrence of osteonecrosis of the jaw (ONJ) using bisphosphonates (BP) in the treatment of osteoporosis (OP).
MethodsWe conducted a systematic literature search in PubMed, EMBASE and the Cochrane Central Register of Controlled Trials up to July 2010, including terms relating to OP, ONJ, and BP (MeSH and free text). We selected meta-analysis, systematic reviews (SRs) and clinical trials (CTs), English or Spanish, including patients >18 years of both sexes with OP treated with BP (intravenous and oral). Furthermore, studies should evaluate the occurrence of ONJ during treatment with BP. We excluded studies that included patients with cancer or diseases other than OP, animal studies and basic science. The selection of articles both by title and 2 independent reviewers conducted a detailed review of the abstracts. We used the modified Oxford Scale (version 2001) to assess the quality of the included studies.
ResultsWe identified 1422 articles of which we included 18 (8 SRs, 8 CT and 2 meta-analysis). Most studies were of good quality and examined the use of BP in middle-aged women with OP. Frequency of ONJ was low.
ConclusionsWe found insufficient evidence to affirm that intravenous or oral BP used exclusively for the treatment of OP lead to a significant risk of ONJ (evidence level 2a, grade B recommendation).
Evaluar sistemáticamente la literatura en relación con la aparición de osteonecrosis de mandíbula (ONM) con el uso de bisfosfonatos (BF) en el tratamiento de la osteoporosis (OP).
MétodosSe realizó una búsqueda sistemática de la literatura en Pubmed, EMBASE y la Cochrane Central Register of Controlled Trials hasta julio de 2010 incluyendo términos relativos a OP, ONM, y BF (mesh y texto libre). Se seleccionaron metaanálisis, revisiones sistemáticas y ensayos clínicos (EC), en inglés o español, que incluían pacientes > 18 años de ambos sexos con OP en tratamiento con BF (por vías intravenosa y oral). Además, los estudios debían evaluar la aparición de ONM durante el tratamiento con BF. Se excluyeron los estudios que incluían pacientes con cáncer u otra enfermedad distinta de la OP, estudios en animales y ciencia básica. La selección de los artículos, tanto por título y abstract como la revisión en detalle, la realizaron 2 revisores de forma independiente. Se utilizó la escala de Oxford modificada (versión del 2001) para evaluar la calidad de los estudios incluidos.
ResultadosSe identificaron 1.422 artículos, de los que se incluyeron 18 (8 revisiones sistemáticas, 8 EC y 2 metaanálisis). La mayoría de los estudios son de buena calidad y estudiaron el uso de BF en mujeres de mediana edad con OP. La frecuencia de ONM fue baja.
ConclusionesNo hemos encontrado evidencia suficiente para afirmar que los BF por vía oral ni intravenosa utilizados exclusivamente para el tratamiento de la OP confieran un riesgo significativo de ONM al paciente (nivel de evidencia 2a, grado de recomendación B).
OP is a disease characterized by decreased bone strength, predisposing an increased risk of fracture.1 Its prevalence increases with age and is a serious public health problem because it can potentially cause devastating results and has a high cumulative rate of fractures. It is estimated that up to 50% of Caucasian women (and 20% of men) over age 50 will have an osteoporotic fracture over their lifespan.2–4 The most relevant fractures are vertebral, forearm and especially the hip. They produce functional impairment and increased morbidity, as well as an increased use of health resources.
Randomized clinical trials (RCTs) have shown that BP increase bone mineral density and reduce the risk of fractures.5,6 Nitrogenated BPs (alendronate, risedronate, ibandronate, pamidronate and zoledronate) are the first therapeutic option for the management of OP. BPs are inorganic pyrophosphate analogs, which are not metabolized to the hydroxyapatite found in bone and remain there for 10–12 years,7,8 and are released in very small quantities during bone remodeling. They inhibit osteoclastic bone resorption by inhibiting farnesyl diphosphate synthetase. Their antiresorptive potency and retention in bone differ depending on the BP.
Moreover, in 2003 the first clinical case of ONJ in a patient treated with BP was published. Since then, there have been over 2400 cases9 generally in cancer patients treated with intravenous BP. Based on case reports or case series, the incidence of ONJ associated with intravenous BP in patients with cancer has been estimated between 1% and 10%.10 The lack of agreement in the estimates of incidence is attributed to differences in methodology when identifying cases and difficulties in accurately quantifying the patients treated with BP. There have also been some cases of ONJ in BP treated OP patients, but the true incidence is unknown. Estimates range from less than 1/100000 to over10 1/10000 cases-year.
We designed this study to estimate the incidence of ONJ in patients diagnosed with OP treated with BP.
Materials and MethodsWe conducted a SR of the scientific literature aimed at estimating the incidence of ONJ in BP treated OP patients.
Search StrategyStudies were identified through a literature search of major databases. For this purpose, an expert documentarian worked and verified them. The terms used to capture and the results are shown in Table I (available in the electronic version of this article). The following databases were screened: Medline (from 1996 to July 20, 2010), EMBASE (1991 to July 20, 2010) and Cochrane Central. We also searched the abstracts of the EULAR and ACR congresses of the years 2008, 2009 and 2010. There were no language restrictions. All references retrieved were handled in EndNote X3 (Thomson Reuters). Finally, a manual search was conducted by reviewing the references of studies included.
Selection Criteria for StudiesThe studies retrieved by the above search strategy were included if they met the following inclusion criteria. Patients had to be ≥18 years with OP of non-malignant etiology and treatment for OP with one of the BP (alendronate, etidronate disodium, ibandronic acid, pamidronate, risedronate and zoledronic acid) or strontium ranelate. We included meta-analyzes, SRs and CT. The comparator group in the RCT could be placebo or active drug. It should analyze the presence of ONJ as a measure of outcome. We excluded animal and basic science studies.
Study Selection, Data Collection and AnalysisTwo reviewers independently examined the titles and abstracts of the articles retrieved with the selection criteria. This process was conducted in 20min sessions. The 2 reviewers extracted the data from studies included using ad hoc standardized forms. All items collected were double and independent. One reviewer entered data forms into a spreadsheets. If, in doing so, the reviewer noticed a discrepancy between the information and the other reviewer, then a consensus was reached by reviewing the original article or by asking a mentor. Items which did not meet all the inclusion criteria or had insufficient data were excluded. To classify quality, we used a modification of the Oxford Centre for Evidence-based Medicine Levels of Evidence11 in which the levels of evidence correspond to: (1a) SR of RCTs with homogeneity, i.e. including studies with comparable results and the same objectives; (1b) individual RCT (with narrow confidence intervals); (1c) proven through clinical practice rather than experimentation; (2a) SR of cohort studies with homogeneity and (2b) individual cohort randomized clinical trials of low quality (<80% follow up); (2c) research studies and ecological “Results”; (3a) case–control studies with homogeneity; (3b) individual studies of cases and controls; (4) case-cohort and low-quality case–control series; (5) expert opinion without explicit critical appraisal, or based on physiology, bench research or “first principles”. Evidence tables were constructed. The meta-analysis was planned just in case there was sufficient homogeneity between the studies included.
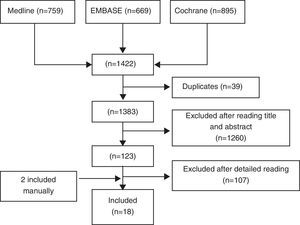
ResultsWe identified a total of 1422 articles, of which we finally included 18 (Fig. 1). In Table II we show excluded studies and reasons for exclusion (available in the electronic version of this article). Of the 18 selected articles we differentiate 8 RCTs9,10,12–17 (see Table 1), 7 double-blind RCTs18–24 and open CT25 (Table 2) and 2 meta-analyzes of RCTs and alendronate5 and risedronate6 (Table 3). Most studies are of good quality and examined the use of BP in middle-aged women with OP.
Articles Selected. Systematic Reviews.
| Study | Population | Selection | ONJ Outcome Measures | Comments |
| Author, yearFollow upType of study | • Inclusion criteria• Exclusion criteria | • Studies• Patients | • Incidence ONJ• Other characteristics | • Quality (Oxford)• Other |
| Filleul,9 20102003–Sep./2009 | • Definition ONJ (Ruggiero, 2006)• Not compliant with definition or duplicate | • 138 articles, 2408 cases ONJ, 61% women• 88% BP iv, 89% cancer• 12% BP oral (77 ALN, 19 IBA)• 67% dental extraction and 7% other factors (dentures, trauma) | • No estimation of ONJ incidence | • 3a• Incidence can be higher, because no non indexed journals were not included |
| Khosla,10 2007Until 2006 | • Patients with BP for OP and ONJ• Minimum 2 years treatment | • Case series• 57 ONJ, more freq in women, 2/3 on mandible | • No estimación de incidencia ONM | • 3a• Incidence unknown, might be subestimated |
| Woo,12 20061996–31/01/2006 | • BP and ONJ, any bone metabolic disease | • 30 case series• 368 cases of ONJ, 94% BP iv and 6% oral (3 Paget, 15 OP with BP oral)• More freq. women (3/2)• 65% Mand, 26% MAX• 60% prior dental extraction | • Estimates incidence for BP iv (3%–10%), but not for OP | • 3a• Suggests marked suppression of bone metabolism as cause |
| Pazianas,13 20071996–Sep. 2006 | • Adult patients with BP for OP and ONJ | • Series of cases (8), observation studies (3)• 26 ONJ (190 Millions prescrip.)87% Wolen• No clear association with time of exposures• Prior intervention or dental trauma 80%• 73% Mand | • No estimate of incidence of ONJ | • 3a• Suppression of bone remodeling→lack of microdamage repair→ONJ |
| King,14 20081950–30/06/2007 | • BP and ONM | • z4 studies (case series, RCT)• 481 ONJ, 58.3% women, 94% cancer, 94% BP iv• 25 OP with BP oral• 20.7% spontaneous• 68.8% prior dental intervention65% Mand, 29% MAX, 6% both | • No estimate of incidence of ONJ | • 3a• Only 1 BD• ZOL in OP: 1 case with RCT, afterward no |
| Hess,15 200801/97–10/07 | • BP and ONM | • 199→37 abstracts+44 manual→81 articles (1 RCT)• 85 patients with OP and ONM• 92% prior dental intervention and 71% active bone drugs | • No estimate of incidence of ONJ | • 3a• Only 1 BD• Cases in patients w/o BP• Other causes than BP |
| Khan,16 2009Until Feb. 2008 | • Diverse articles | • 92 studies: 34 reviews, 16 cases, 18 case series, 10 editorials, 2 observation prospective studies, 2 surveys, 8 letters and 2 guidelines• ONJ: 1.861 cases, oral BP 4%–5% of total, in OP<1/100000 cases | • No unified definition of ONJNo estimate of incidence of ONJ | • 3a• Cases w/o BP. Dif Dx: LMSU• Other risk factors: Rt, Qt, GC• No relationship between ONJ and BP oral |
| Palaska,17 20092003–2008 | • BP and ONM• No if prior Rt | • 112→71 case series, 18 in OP• 767 with BP iv (388 ZOL, 165 PAM, 214 both)• 73% prior dental intervention | • No estimate of incidence of ONJ | • 3a• Only 1 BD• Cases of ONJ w/o BP• Other risk factors in addition to BP |
ONJ: osteonecrosis of the jaw; OP: osteoporosis; BP: biphosphonates; iv: intravenous; tto: treatment; freq: frequent; Mand: mandible; MAX: maxillary; RCT: randomized control trial; Rt: radiotherapy; Qt: quemotherapy; GC: glucocorticoids; ZOL: zolendronic acid; ALN: alendronate; IBA: ibandronate; PAM: pamidronate.
Selected Articles. Controlled Clinical Trials.
| Study | Population | Intervention | ONJ Outcome Measures | Comments |
| Author, yearsFollow upType of study | • Inclusion criteria• Exclusion criteria | • Studies• Patients | • Incidence ONJ• Other characteristics | • Quality (Oxford)• Others |
| Black,18 200660 monthsRCT double blind placebo control | • 1099 women with OP, mean age 73 years±5 | • ALN 10mg/d• ALN 5mg/d• Placebo• Cointervention: calcium+vit. D | • No cases ONJ | • 1b |
| Voskaridou,19 200612 monthsECA double blind placebo control | • 66, 66% women, mean age 44 years±11• Patients with thalassemia and OP | • ZOL iv 4mg/6m• ZOL iv 4mg/3m• Placebo• Cointervention: calcium | • No cases ONJ | • 2a |
| Black,20 200736 monthsECA double blind placebo control | • 7765 (3889 ZOL), posmenop OP mean age 73 years±5 | • ZOL iv 5mg/12m• Placebo• Cointervention: calcium+vit. D | • ZOL: 1 case (0.000026%)• PLB: 1 case (0.000026%) | • 1b• 7 cases ON hip or knee, 4 ZOL and 3 placebo |
| Lyles,21 200760 monthsECA double blind placebo control | • 2127, 76% women, mean age 74 years±9, w hip fracture <90 days, no prior ranelate tto or fluor | • ZOL iv 5mg/12m• Placebo• Cointerventions calcium+vit. D | • No cases ONJ | • 2c |
| Eisman,22 200824 monthsRCT double blind placebo control | • 1395 posmenop women w OP, mean age 66 years±6 | • IBN iv 2mg/2m• IBN iv 3mg/3m• IBN vo 2.5mg/d• Placebo | • No cases ONJ in 1089 patients with IBN for 2 years | • 2c |
| Reid,23 200912 monthsECA double blind placebo control | • 545, 68% women, mean age 53 years±14 in steroid tto | • ZOL iv 5mg/12m• RIS vo 5mg/d• Placebo• Cointervention: calcium+vit. D | • No cases ONJ | • 1b |
| Orwoll,24 201012 monthsRCT double blind placebo control | • 135 men with OP no tto, mean age 64 years±11• Stratified for testosterone | • IBN orally 150mg/m• Placebo | • No cases ONJ | • 2c |
| Jeffcoat,25 200636 monthsCT open | • 50 posmenop women and OP who merited dental imlants (220) | • ALN 70mg/week• RIS 35mg/weekPlacebo | • No cases ONJ or differences in implant loss | • Jadad 1 |
ONJ: osteonecrosis of the jaw; OP: osteoporosis; mg: milligram; m: month; iv: intravenous; vo: orally; tto: treatment; freq: frequent; vit. D: vitamin D; RCT: randomized clinical trial; ZOL: zolendronic acid; ALN: alendronate; IBA: ibandronate; RIS: risedronate; PLB: placebo; fract: fracture.
Articles Selected. Metaanalysis.
| Study | Population | Intervention | ONJ Outcome Measures | Comments |
| Author, yearFollow upType of study | • Inclusion criteria• Exclusion criteria | • Studies• Patients | • Incidence ONJ• Other characteristics | • Quality• Others |
| Wells,5 2008 a1996–2007SR-MA | • Control RCT>1 year, ALN, prevention of fractures posmenop women• <1 year, no RCT, no control | • 1203→85→11 RCT: 12068 patients→6543 received ALN for 1–4 years→>15000 patients year | • No cases of ONJ | • 2nd• ONJ was not outcome of the 11 RCT in the MA |
| Wells,6 2008 b1966–2007RS-MA | • Control RCT>1 year, RIS, posmenop women and OP• <1 year, non randomized, no control, duplicated | • 419→36→7 RCT: 14049 patients→9303 OP with RIS from 1 to 3 years→>20000 persons/year | • No cases of ONJ | • 2nd• ONJ was not outcome of the 7 RCT in the MA |
ONJ: osteonecrosis of the jaw; OP: osteoporosis; BP: biphosphonates; MA: metaanalysis; ALN: alendronate.
In connection with the SR sample, one12 identified, until January 2006, 368 cases of ONJ with 15 (4%) occurring in patients with OP treated with oral BP. Another,13 based on extensive case findings, case series and observational studies in OP patients treated exclusively with BP, from 2003 to September 2006, identified 26 cases of ONJ in 11 studies, reflecting a very low incidence of ONJ compared to 190 million estimated prescriptions of BP for OP. Patients were mostly women (87%), with a highly variable age (39–83), and usually had a history of dental surgery or trauma (80%).
In 2007 a report of the American Society for Bone and Mineral Research (ASBMR) was published on BP-associated ONJ in which there was a SR of ONJ in OP patients treated with BP orally, which contributed a total of 57 cases, most associated to intravenous BP (iv).10 The report suggested that the actual incidence may be higher than those published so far, due to confounding factors.
During the following years, there was a progressive increase in cases of ONJ in patients receiving BP for the treatment of OP. Until September 2009, another review9 found 2408 cases of ONJ associated with BP, of which 178 corresponded to patients with OP.
Based on the results of these SR,9,10,12–17 it must be considered that in less than 25%, ONJ occurs spontaneously, is most frequently localized in the mandible (65%–70%) and can simultaneously affect both maxillary bones (5%–10%). Factors associated with its occurrence were mainly dental procedures and to a lesser extent, defective dentures, trauma, bone exostosis, drug therapy (corticosteroids), and so on. For some studies there was no clear time dependence of exposure,13 but others showed a minimum of 2 years and a mean of 4.6 years of exposure to BP for the development of ONJ.10,17 About 80% of patients were over 60 years and were predominantly female.13 There have been no cases of ONJ associated with the use of strontium ranelate in OP.
Controlled Clinical TrialsOnly some of the latest clinical trials referred to jaw osteonecrosis as a possible adverse event of OP treatment with BP. In general, clinical trials measure efficacy and safety of at least 1 year in duration. Due to frequency and volume of patients we included those performed with zoledronate. Treatment for 5 years with 5mg iv annual zoledronate vs placebo in 2127 patients over 50 who had had a hip fracture in the last 3 months did not show any cases of ONJ.21 Neither reported any cases of ONJ, although active surveillance was performed in 66 patients with thalassemia and OP treated for 1 year with 2 different regimens of iv zoledronate (4mg/6 months or every 3 months) vs placebo19 or in 545 patients with glucocorticoid-treated OP for one year with a dose of 5mg zoledronate vs iv. 5mg oral risedronate.23 Finally, neither showed any cases in a RCT conducted in 7765 women between 65 and 89 years of age with postmenopausal OP, which studied the efficacy and safety of annual iv zoledronate 5mg vs placebo.20 The occurrence of ONJ was not a primary endpoint of the study, but with the ongoing study it was decided to assess maxillofacial adverse events in an objective and independent manner to identify a possible association. After reviewing the database for adverse events, 101 patients in the zoledronic group and 127 patients in the placebo group who had maxillofacial potential adverse events that met the criteria for an investigation were identified and subsequently attributed to ONJ. The independent adjudication committee consisted of 5 experienced specialists and decided that 2 patients (one from each treatment group) had an event that met the definition criteria of ONJ. In both cases there was a delay in healing after surgery.26
ONJ was also a primary endpoint in the trials with ibandronate. In 2 of them there was no communication of any cases of ONJ. One compared 2 intermittent iv regimens with daily oral ibandronate for 2 years in 1395 women between 55 and 80 years with posmenopausal22 OP and the other studied the efficacy and safety of ibandronate (150mg PO month) vs placebo in healthy young men with OP.24
The FLEX18 study compared the effect after treatment and was discontinued in 1099 women who had been treated with alendronate in the FIT study for 5 years. Although there was a significant number of women treated with alendronate for many years, there were no cases of ONJ. Among women who discontinued therapy after 5 years and followed until 10 years after treatment stop, there were no differences in the occurrence of ONJ.
In most cases of ONJ reported in BP treated OP, patients had undergone dental procedures of different kinds. An open clinical trial compared the outcome of 220 dental implants placed in 50 women with posmenopausal OP.25 Half were treated with weekly BP (alendronate 70mg or risedronate 35mg) and half received no treatment. The main endpoints were loss of implants and the occurrence of ONJ. There were no cases of ONJ or differences in the loss of implants.
Meta-analysisFinally, there also were no cases of ONJ associated with BP in the meta-analyses of controlled clinical trials lasting at least one year for postmenopausal OP treatment with and risedronate and alendronate.17,18
DiscussionThere have been reports of ONJ in patients treated with BP and, more recently, with denosumab. It has also been described in individuals who have never taken these drugs.
Initially, cases were reported in patients with neoplastic disease who had received large doses of intravenously BP. The rising incidence of ONJ in patients treated OP BP in recent years caused alarm in the scientific community and the general population. Given the paucity of evidence on this controversial issue, different societies have published recommendations based on the opinion of experts.27,28
The incidence of ONJ in the general population is not known accurately and reliably. In patients receiving BP to treat OP, estimates range, according to the ASBMR,10 from less than one case per 100000 patients/year of treatment with BP, drawn from the epidemiological surveillance and a German retrospective and one ≥case per 10000 inhabitants, based on an Australian study with many methodological limitations performed by postal survey. When analyzing most of the RCTs of BP for OP, ONJ was not associated with BP, so that compatible cases were not collected. By contrast, in studies with zoledronic acid in postmenopausal OP and corticosteroid-induced OP, no possible cases of ONJ were collected. In the study of postmenopausal OP (HORIZON) only 2 cases of ONJ were seen, one in each treatment group (placebo and zoledronic acid); therefore it was concluded that zoledronic acid at the doses used to treat OP (5mg iv year) does not increase the risk of ONJ.21 Two large well designed case–control studies also found no association between the use of oral BP and the risk of ONJ.29,30
Although potential mechanisms are known, the pathophysiology of ONJ is not clear.31 Some data support a dose–response relationship between ONJ and BP. A high dose of BP and its potency and duration of antiresorptive therapy are associated with the appearance of ONJ. In fact, the estimated incidence is much higher in patients receiving high-dose iv BP for malignancy (1%–10%) than in patients treated with BP (vo or iv) for OP, but did not rule out factors associated with neoplasms that contributed to the development of ONJ. The emergence of new cases of ONJ with another potent inhibitor of bone resorption such as denosumab suggests that the suppression of bone turnover plays a role in this complication. According to Compston,31 multiple local and systemic risk factors seem to be involved in the pathogenesis of ONJ associated with BP, but the exact contribution of each is unknown. In the SR we found that ONJ in patients with OP treated with BP is located more frequently in the mandible and in most cases its appearance was associated with previous dental work. In some studies we found no clear dependence on exposure time, but in others a minimum of 2 years of exposure to BP was established for the development of ONJ. Different societies believe that patients who have been treated <3 years with BP for OP can undergo dental treatment without an increased risk of developing ONJ.10,27,28 Finally, most patients who developed ONJ were over 60 years and predominantly female and had concomitant diseases and treatments.
In SR limited to other clinical trials with ONJ as an outcome, we have not found enough evidence to say that IV or oral BP, or used exclusively for the treatment of OP, conferred a significant risk of ONJ (evidence level 2a, grade B recommendation). However, we note that, in general, patients included in clinical trials do not represent the general population, since they often have less and concomitant comorbidity.
As of September 2009 178 cases of ONJ have been published. Surely, not all cases occurred or are all published. In any case, the reported incidence in patients receiving BP is very low compared with the prevalence of OP, compared with the risk of fractures and BP volume requirements.
In summary, the correct use of BPs in patients at high risk for fracture so far seems to offer a risk/benefit ratio favoring their use in this population. The optimal duration of treatment remains to be defined, considering that the incidence of ONJ appears to be associated with it and whether the drug accumulated in bone (12 years) maintains antiresorptive capacity.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Chamizo Carmona E, et al. Revisión sistemática de la literatura sobre la osteonecrosis maxilar con el uso de bisfosfonatos en pacientes con osteoporosis. Reumatol Clin. 2012. http://dx.doi.org/10.1016/j.reuma.2012.05.005.