Platelet rich plasma (PRP) is a novel therapeutic tool that has revolutionized the world of sports medicine and trauma due to therapeutic success shown in the media. Subject to ongoing debate, the PRP is outlined along a spectrum of musculoskeletal therapies with many qualities that make it ideal for use in the rheumatology: effectiveness, safety, easy handling and low cost. Is PRP a product of marketing? Or, conversely, is an interesting tool to consider in the armamentarium of the rheumatologist. In the following review we will analyze in detail its principles, preparation, and management regimes. We will reflect on potential adverse effects and, finally, there will be a critical analysis of the scientific evidence that supports its potential use in the rheumatology clinic.
El plasma rico en plaquetas (PRP) es una novedosa herramienta terapéutica que ha revolucionado el mundo de la medicina deportiva y la traumatología debido a éxitos terapéuticos mediáticos en deportistas de élite. Sujeto a continuo debate, el PRP se perfila en el espectro de las terapias musculoesqueléticas con múltiples cualidades que potencialmente lo hacen idóneo para su uso en la consulta de reumatología: efectividad, seguridad, fácil manejo y bajo coste. ¿Es el PRP un nuevo producto de la mercadotecnia? o, por el contrario, es una herramienta con fundamento que debe incluirse en el arsenal terapéutico del reumatólogo. En la siguiente revisión se repasarán en detalle su fundamento, preparación y regímenes de administración. Se reflexionará sobre potenciales efectos adversos y, por último, se realizará un análisis crítico de la evidencia científica que avala su posible uso en la consulta de reumatología.
Platelet rich plasma (PRP) is a novel therapeutic tool of autologous nature that has emerged strongly in recent years due to successful therapeutic use in elite1 athletes. Famous professional football players, Tiger Woods and Rafael Nadal attribute, in part, their “miraculous” recoveries to the employment of this enigmatic treatment dubbed the “PRP phenomenon.” PRP treatment is little known but quite prominent in rheumatology, orthopedics and sports medicine congresses in which, despite the controversy surrounding it, has gradually awakened growing interest because of its supposed effectiveness and apparent lack of side effects.2 Its therapeutic target eminently comprises chronic tendinopathy and enthesopathy, although the range of indications is constantly expanding and has been successfully used in many ailments, including knee osteoarthritis. Its low cost, ease of use, usefulness in pathological processes elusive to conventional treatments and apparent safety make it a seductive alternative to consider in the therapeutic armamentarium of the rheumatologist.
The following review attempts to analyze what has been called the “PRP phenomenon’; I will review in detail the rationale for use, preparation and management regimes. I will also discuss and critically analyze its safety profile and the scientific evidence supporting its use and consider its possible usefulness in rheumatology.
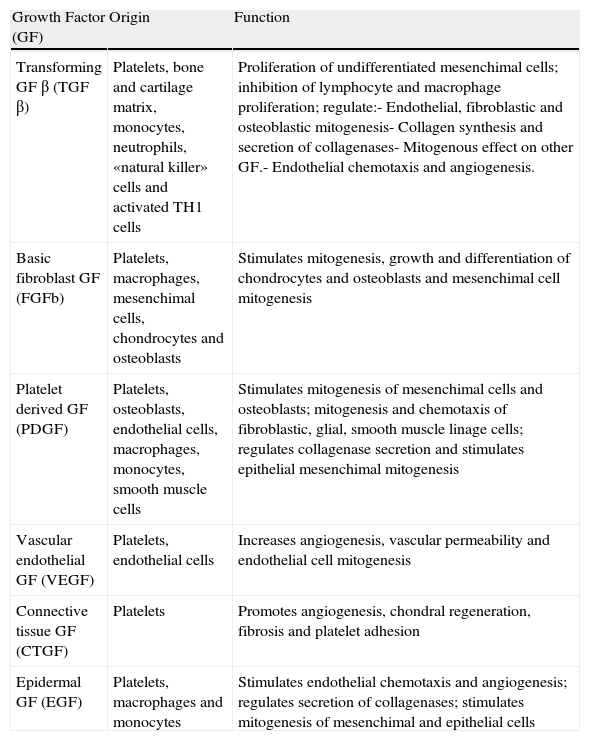
BackgroundPRP owes its therapeutic interest to the crucial instrumental role of platelets in the wound healing and tissue repair processes. This role is not related to the repairing properties of the platelets themselves but, rather, to growth factors (GF) released by its α granules, which possess multiple regenerative properties (Table 1). Tissue wound repair is a complex process in which a variety of cellular functions such as chemotaxis, angiogenesis, cell proliferation, extracellular matrix formation and the “cleansing” macrophage coexist, sequentially and covertly. These functions form a complex in which three relatively distinct phases are classically distinguished: inflammation, proliferation and remodeling.3–7 All GF content of the PRP is involved in the phases described, but all of their functions are still unknown. It is speculated that some of them play a role,4 but it is conceivable that each individual prominence varies depending on the type of tissue wound (ruptured, inflammation, degeneration, etc.) and the type of tissue (tendon, muscle, bone, etc.).
Growth Factors Contained in Platelet Rich Plasma.
| Growth Factor (GF) | Origin | Function |
| Transforming GF β (TGF β) | Platelets, bone and cartilage matrix, monocytes, neutrophils, «natural killer» cells and activated TH1 cells | Proliferation of undifferentiated mesenchimal cells; inhibition of lymphocyte and macrophage proliferation; regulate:- Endothelial, fibroblastic and osteoblastic mitogenesis- Collagen synthesis and secretion of collagenases- Mitogenous effect on other GF.- Endothelial chemotaxis and angiogenesis. |
| Basic fibroblast GF (FGFb) | Platelets, macrophages, mesenchimal cells, chondrocytes and osteoblasts | Stimulates mitogenesis, growth and differentiation of chondrocytes and osteoblasts and mesenchimal cell mitogenesis |
| Platelet derived GF (PDGF) | Platelets, osteoblasts, endothelial cells, macrophages, monocytes, smooth muscle cells | Stimulates mitogenesis of mesenchimal cells and osteoblasts; mitogenesis and chemotaxis of fibroblastic, glial, smooth muscle linage cells; regulates collagenase secretion and stimulates epithelial mesenchimal mitogenesis |
| Vascular endothelial GF (VEGF) | Platelets, endothelial cells | Increases angiogenesis, vascular permeability and endothelial cell mitogenesis |
| Connective tissue GF (CTGF) | Platelets | Promotes angiogenesis, chondral regeneration, fibrosis and platelet adhesion |
| Epidermal GF (EGF) | Platelets, macrophages and monocytes | Stimulates endothelial chemotaxis and angiogenesis; regulates secretion of collagenases; stimulates mitogenesis of mesenchimal and epithelial cells |
PRP is defined as a plasma fraction containing a higher concentration of platelets than the peripheral blood. Some authors are more precise and consider PRP platelet concentrations to be approximately five times above normal.8 Actually, it is a plasma aliquot of 20–30ml of peripheral blood centrifuged at 3200rpm for 15min. The result is approximately 2–3ml of platelet-rich plasma with varying concentrations, usually about 1×106 platelets. Said aliquot can be activated with thrombin or calcium chloride for future therapeutic usefulness. When PRP is intended to treat soft tissue injuries most authors consider prior activation not necessary, because this is produced in situ in contact with the tendon collagen or fibrillar9 breakage clot itself. When PRP is used to facilitate integration of bone implants, or when used for the treatment of knee osteoarthritis, some activation is usually preferred giving it a gelatinous consistency that facilitates its surgical use.10
The doses and administration regimes vary depending on the condition being treated. In the case of chronic tendinopathy, the literature seems to agree on a single administration for epicondylitis, Achilles tendinitis and plantar fasciitis.9,11,12 However, it may be used on three consecutive occasions (one every two weeks) in the case of a chronic patellar tendinopathy or “jumper's knee”.13 There are some authors who frequently use PRP for the treatment of impingement syndrome, especially when it is chronic or refractory. However, no reliable information is available to provide evidence of a specific dosing schedule. Recently the usefulness of PRP for treating osteoarthritis of the knee has been reported. Most authors agree that the ideal number of infiltrations in these cases should be 3, but there is no unanimity about the dosing interval, with good results both on a weekly, as well as triweekly basis.14,15
SafetyThe autologous nature of PRP appears to be the main argument for its safe tolerance on infiltration, rarely producing mild local inflammatory reactions. There have also been reports of postpuncture infection data that may be able to relate to the antimicrobial effect of PRP as suggested by some authors.16 Adherents to PRP say repeatedly that no undesirable effects exist because it is an autologous product in nature. The truth is that these statements are based only on their own experiences, because there are neither safety studies, nor authoritative information from clinical trials. Conceivably, the autologous nature of PRP provides therapeutic tolerance, but tolerance does not mean safety. The therapeutic effect is achieved by concentrating PRP's massive amounts of GF in the damaged tissues. As mentioned, GF promote multiple cellular functions that well modulated may result in an acceleration of tissue repair process. Tissue repair is not the only process in which these factors contribute decisively. The endothelial growth factor (VEGF), the basic fibroblast growth factor, hepatocyte growth factor (HGF), and insulin growth factor are particularly important factors in the growth of certain tumors because of their angiogenic high potential. Some GF have even exhibited oncogenic potential themselves, capable of promoting clonal expansion of previously mutated cells, facilitating their immortalization cycle by inhibiting apoptosis.17–19 These properties of GF, promoting the generation and perpetuation of tumors, although disturbing are only described in experimental animals, and there is no evidence so far linking the therapeutic use of PRP in humans with carcinomatous transformation.20
It has recently been demonstrated that certain GF favor proliferation or activation (in situ and peripheral) of circulating osteogenic progenitors or “COP cells”.21 These recently described undifferentiated cells are known as osteochondral progenitors that are definitely involved in extraskeletal bone formation, as occurs in postarthroplasty heterotopic ossification22 or valve calcifications. It seems reasonable that, in the presence of a suitable microenvironment in the wound tissue, exaggerated GF concentrations contained in the PRP may promote recruitment and activation of COP supranormal cells in the infiltration area with capacity to develop heterotopic calcification or intra level ossification of soft tissue. To date, these reflections do not stop from being mere speculation since no complications have been reported in the literature as such.
Preclinical EvidenceTendon and MuscleMost of the preclinical evidence supporting the benefit of PRP in tendinopathy is based on its supposed ability to stimulate tissue repair process. Cell cultures provide evidence that PRP is involved in the different phases of the repair process. In the inflammatory phase, the GF contained in PRP can mobilize circulating inflammatory cells into the wound.23 They also stimulate tenoviocytes to secrete collagen, VEGF and HGF, all of them reparative24 process accelerators. In the proliferative phase, PRP is capable of stimulating the proliferation and division of almost all types of mesenchymal cells (osteoblasts, fibroblasts, chondrocytes or tenoviocytes) and also multipotential25 stem cell.26 In the final phase of remodeling, PRP facilitates newly formed collagen maturation and apoptosis of excess cells.27 Taken together, all of these evidences involve PRP definitely in the tissue repair process, but do not clarify whether this involvement translates directly into acceleration, and even less when it favors faster and more efficient healing, as proposed by some authors. Some of these questions have been clarified by certain animal models. In murine models with partial section of the Achilles tendon, PRP has been shown to be associated with a faster consolidation of the tendon callus, which is also mechanically efficient.28,29 Part of this effect could be due to its ability to accumulate circulating proinflammatory cells in the tendon wound.23 In muscle, some models also have observed a benefit of PRP in the myofibrillar repair process. In a model of breaking-strain of the tibialis anterior in rats, PRP seems to significantly shorten recovery times in those lesions that require myogenesis for recovery, directly involving them in the process of muscular neoformation.30 None of these studies have yet determined the properties that each GF individually has in these two different microenvironments. The response to PRP in animal models of inflammatory tendinopathy31 has also been described, more in line with the reality of clinical rheumatologists.
Bone and JointsHistorically the first PRP therapeutic applications began in the field of dental surgery for compacting maxillofacial implants and bone32 sealant. These osteointegrating properties caught the attention of some orthopedic surgeons who saw PRP as the ideal adjuvant to shorten the recovery of certain surgeries.33 This osteoinductive and sealant effect is still controversial, but the most consistent and valuable for many of its advocates. The truth is that the composition of PRP's many factors individually has shown a potent bone anabolic effect. The osteoinductive potential of platelet derived growth has been confirmed in more than one study so some authors have speculated on its clinical use (especially the BB isoform) in osteoporosis and consolidation of fracture34 calluses. One of the best-known properties of the epidermal growth factor (EGF) is its ability to expand totipotent stem cell populations. EGF is also able to differentiate these cells to the osteoblast lineage, thereby favoring remarkable osteogenesis.35,36 The fibroblast growth factor (FGF) is a classic and very powerful stimulator of the differentiation and activation of osteoblasts and has bone anabolic potential which is due in large measure to the Wnt37 pathway activation. In rabbits, FGF has been used successfully for the treatment of hip osteonecrosis, avoiding collapse not only of the femoral head, but also the development of secondary38 coxarthrosis. In humans, FGF significantly accelerates the formation of bone callus, shortening tibial39 osteotomy patient's time to discharge. The transformative growth factor β (TGF β) is a GF widely involved in bone and chondral40 anabolism. However, the TGF β superfamily is a complex of factors and receptors which, under various circumstances, can be converted into powerful stimulators and also osteoclast determinants associated to, for example, myeloma bone aggressiveness and multiple skeletal41,42 metastases.
The set of all such evidence suggests that, by virtue of its composition, PRP meets the right qualities to play a powerful osteoinductive role, capable of accelerating consolidation or osteointegration of fractures quickly and efficiently43,44 as well as of different types of implants.45 However, the literature is not unanimous in weighing these hypothetical beneficial properties of PRP.46–48 The relative concentrations of each GF upon activation, the dosing schedule, the type of injury and the microenvironment are determinants that seem to alter the osteoinductive qualities of GF which may be translated into the real clinical benefits of PRP.
The use of PRP in joints is relatively recent and has scant preclinical literature. Regarding degenerative arthropathy, there is only one work that analyzes the effect of PRP in knee osteoarthritis in rabbits. In this study, the authors histologically examined knees of rabbits treated with PRP-impregnated microspheres, observing a reduction in the progression of osteoarthritis with respect to controls. Using complementary cell cultures, the authors concluded that this effect was due to the metabolic stimulation of chondral matrix synthesis through glucosaminglycans.49 Regarding inflammatory arthritis, a paper has just recently been released, of particular interest to rheumatologists, which analyzes the effect of PRP in a porcine model of antigen-induced rheumatoid arthritis. In this study, intra-articular treatment with PRP of arthritic knees resulted, histologically and immunohistochemically, in a significant attenuation of the inflammatory changes characteristic of the disease, both at the synovial and50 cartilage levels. These results contrast to some extent, with the recently proinflammatory effect attributed to platelets in rheumatoid arthritis and other inflammatory arthropathies.51
Clinical EvidencePlatelet Rich Plasma and Subacromial TendonitisThe use of the PRP in the subacromial syndrome and all variants lacks, to date, studies that evaluate it as a single treatment. The only evidence mentions its use as an adjuvant in decompressive and reconstructive surgery of the rotator cuff. In this type of surgery, PRP improves the results of conventional reconstructive surgery and shortens recuperation time.52,53
Platelet Rich Plasma and EpicondylitisEpicondylitis is a limiting tendinopathy, with a clear tendency to become chronic, which has a random, often partial response to glucocorticoid infiltrations and physiotherapy. Some authors have investigated the effectiveness of PRP in this disease because of the potential remedial effect observed in animal models. The pioneering essay that analyzes for the first time the effect of PRP on epicondylitis dates from 2006. This is a pilot, local anesthetic-controlled study, performed on 19 patients with refractory epicondylitis9 who were proposed surgery. In this study, the effect of a single injection of PRP on pain (Visual Analog Pain Scale [VAS]) and functional capacity (Mayo Elbow Score modified) was analyzed. At 8 weeks, patients referred an improvement of 60% (16% in controls), which after two years ended up being 93%. No adverse effects were reported. More recently, an open study with 30 patients also analyzed the role of PRP as salvage therapy in epicondylitis.54 As in previous studies, a single dose of PRP was administered and its effect was evaluated annually on pain (VAS) and functional capacity (American Shoulder & Elbow Surgeons outcome instrument). It reported a 25% improvement in VAS in ≥90% of patients, finding no adverse effects. The findings of the two studies ponder the clinical utility of PRP in patients with refractory epicondylitis. Remember that the design of both had some biases that limit the value of their findings. The main limitation lies in the small number of patients treated in both cases, to which the lack of randomization and blinding in a randomized controlled retrospective manner must be added. At the beginning of 2010 the first quality clinical trial was published, leading to less vulnerable conclusions on the role of PRP in patients with epicondylitis.55 It was a comparative clinical trial, controlled with corticosteroids, randomized, double-blind study on 100 patients with chronic epicondylitis who were followed one year. It analyzed the effect of a single injection of PRP in pain and improvement of functional capacity of the elbow, using corticosteroid infiltration as control. A VAS decreased in the Disabilities of the Arm, Shoulder and Hand score (DASH) more than 25% was considered as a measure of response to treatment. In the PRP group, it was reported by 73% of respondents, while in the group of patients who received corticosteroids only 50% reported the outcome. In this study no adverse effect was reported. The authors concluded that PRP significantly exceeded the clinical benefit obtained by corticosteroids. However, the clinical benefit achieved by corticosteroids in this work is directly influenced by the fact that all the patients included in the trial must have had evidence of pre refractoriness to local injections of corticosteroids. This bias prevents a true comparative analysis as it greatly favors PRP in comparison with steroids. Additionally, the results of this study support the concept reported in the previous 2, on the analgesic properties of the PRP in the treatment of epicondylitis, without presenting clinically significant adverse effects.
Platelet Rich Plasma and Plantar FasciitisPlantar fasciitis is another common problem in rheumatology and, like the rest of tendinopathy, also tends to chronicity. The only evidence that analyzes the effect of PRP on plantar fasciitis dates from 2004 and is the first study that applies PRP to any type of tendinopathy. It is a series of 9 patients, followed for a year, with plantar fasciitis refractory to NSAIDs, immobilization, physical therapy and corticosteroid infiltration, given a single ultrasound-guided injection of PRP in the central and medial calcaneal fascicles. It evaluated the improvement in pain and ultrasound pattern changes. At follow-up, 8 of the 9 patients were asymptomatic but one of them required a second dose. The ultrasound thickness decreased at 3 months (on average) 1.2mm in the central and 2.3mm in the medial bundles. The evidence provided by this work seems worthy of further study, but it is still anecdotal given the small number of patients treated and the biases inherent in a retrospective design and open design.
Platelet Rich Plasma and Knee OsteoarthritisThe application of PRP to treat osteoarthritis of the knee can be considered a relatively new therapeutic indication that focuses undoubtedly on the most current clinical research. This new indication has led to multiple studies in the past 3 years, both controlled and open. Among them, one highlight is a retrospective study of 60 patients with osteoarthritis of the knees, followed for 5 weeks.14 This study compared the therapeutic effect of PRP with high molecular weight hyaluronic acid (HMWHA). The authors treated 30 patients with an infiltration weekly (for 3 weeks) of activated PRP and a similar number with a single dose of HMWHA. Respondents were considered those who decreased the WOMAC pain score ≥40% from baseline. The authors found 33% responders with PRP and 10% with hyaluronic acid at the end of follow-up. Such a low percentage of responders to viscosupplementation is surprising, documented in the literature with much higher response rates than those in this trial.56 Undoubtedly, a very poor response from the control group favors PRP in the comparative analysis. Probably, this analysis would have been more truthful and more clinically relevant if the patients had been tracked longer in order to fully develop the potential HMWHA accredited analgesic efficacy.57 In favor of the results of this study it should be noted that it was the first to specify the relative concentrations of GF contained in the infiltrated plasma, and also it is the only paper published that explicitly uses leukocyte free PRP, a very important fact for some authors.58 Very recently a new study, with a larger number of patients, again compares the effect of PRP with hyaluronic acid. This is a new comparative trial on 150 patients with osteoarthritis of the knees, followed for 6 months. It establishes three treatment arms with 50 patients each: activated PRP (infiltration every 21 days), HMWHA (single dose) and low molecular weight hyaluronic acid (LMWHA) (single dose). It analyzed the effect on the visual analogue scale of quality of life related to health status (EQ VAS) and knee function using the International Knee Documentation Committee index (IKDC). At follow-up, the authors reported better response rates in PRP-treated patients than in those treated with AHAPM or AHBPM (P<.005). In all groups, the response was better in younger patients and in those who had a more recent onset osteoarthritis. As in previous studies, a comparative analysis was not entirely fair and in this case, the AHBPM comparison was not clear. The use of infra treatment therapeutic dose control, as in this case, for AHBPM,59 favors the intervention to be analyzed (PRP). Besides this bias, lack of randomization and blinding lack credibility and, on the other hand, employs rare outcome measures and doubtful discriminative ability for osteoarthritis of the knee, such as IKDC, for example, as it is an index originally designed for the analysis of knee60 ligamentous instability.
Some series of patients with osteoarthritis of the knees, successfully treated with PRP have also been reported in recent years. The largest number belongs to a Spanish group that included 261 patients treated with three injections of activated PRP every 15 days (infiltrated volume not detailed), which was followed for 6 months.61 VAS, WOMAC (pain, stiffness and functional class), and SF-36 Lequesme (baseline and 6 months) were assessed. In their results, the authors reported improvement in all outcome measures, with percentages (relative to baseline) ranging from 8.4 in SF-36 and 30.7 in the WOMAC pain subscale. Another series with a longer follow-up, included 100 patients with osteoarthritis of the knee treated and followed for a year.15 In this work, which also employed 3 doses of activated PRP administered every three weeks, the authors found a significant improvement in VAS and EQ IKDC that, although more marked in the first 6 months, remained for a year. As in other studies of the same group, patients with the most benefit were young and had a less evolved osteoarthritis. Finally, one last article is worth mentioning that evaluates the effect of PRP in 14 patients with refractory prosthetic gonarthrosis62 proposed for replacement. It included patients with osteoarthritis of the knee refractory to conservative treatment (NSAIDs, rehabilitation, acupuncture, corrective splints, injections of corticosteroids or hyaluronic acid) that were treated with 3 doses of activated PRP (one per month). An effect was observed one year after infiltration on the VAS and the Knee Injury & Osteoartrhitis Outcome measure. The authors observed a linear improvement of VAS and Knee Injury & Osteoartrhitis Outcome in 60% of patients at follow-up. Although 40% of patients did not improve, none had to undergo surgery during the study.
Significant adverse effects were not reported in the five above mentioned studies, except for local postpuncture pain.
ConclusionsPRP is a novel therapeutic tool that has arguments for and against its use in the rheumatologist's therapeutic spectrum. Proponents point out to, among its highest virtues, its autologous nature in guaranteeing an excellent safety profile. However, in the reviewed literature, there are no safety studies and clinical trials that can permit such conclusions to be drawn. Series of patients and published works provide information about adverse local reactions. While tolerance to PRP infiltration is excellent as is its short-term safety, this profile in the long-term is still incomplete or at least uncertain.
The supposed effectiveness of PRP in various diseases has not been studied in clinical trials of proven quality. The studies supporting its effectiveness have a level of evidence under multiple biases penalized for both design and implementation. However, the results reported so far are unanimous in stating that PRP, if not effective, is useful in the treatment of epicondylitis and knee osteoarthritis. Among a number of specialists there may be sufficient evidence to justify the use of PRP. They may argue that medicine (until recent years) has progressed and grown outside the orthodoxy of clinical trials, sustained by a less demanding scientific rigor than that reviewed here. They are right. For another great part of clinicians, this evidence will be inadequate and will not neutralize the logical suspicion that a treatment prescribed for multiple conditions that have nothing in common produces, protected by an aura of safety that transcends the strictly scientific. It may be more appropriate not to be seduced by fashion prejudices nor dwell on academics, substantiating the value of this therapy through quality clinical trials that to date do not exist, and seem indispensable to properly develop the therapeutic potential of PRP. This work will require the measurement of many important aspects of the therapeutic usefulness that are still confusing such as collection, processing or activation. Additionally, this will help formalize the doses, volumes and more appropriate management schemes, establish guidelines, and especially more rigorously evaluate its potential safety issues.
Rheumatologists, as leaders in medical pathology of the musculoskeletal system, should enforce judgment and properly size up this novel treatment, as other specialists in the field have already done.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of InterestThe author declares no conflict of interest.
Please cite this article as: De La Mata J. Plasma rico en plaquetas: ¿un nuevo tratamiento para el reumatólogo? Reumatol Clin. 2012. http://dx.doi.org/10.1016/j.reuma.2012.05.011.