Tjalma syndrome or pseudo-pseudo Meigs’ syndrome is a clinical condition characterized by pleural effusion, ascites and elevated CA-125 with no associated benign or malignant ovarian tumor in a patient with systemic lupus erythematosus (SLE). Tjalma described the first case of a patient with SLE, pleural effusion, ascites and elevated CA-125. We report the first case in a 14-year old patient who presented with ascites and pleural effusion refractory to treatment and elevated CA-125, in the absence of an ovarian tumor, that warranted aggressive management.
El síndrome de Tjalma o pseudo-pseudo Meigs es una entidad clínica que se presenta con derrame pleural, ascitis y elevación de CA-125 sin asociación a tumor ovárico benigno o maligno en un paciente con lupus eritematoso sistémico (LES). Tjalma describió el primer caso de un paciente con LES, ascitis, derrame pleural y elevación de CA-125. Presentamos el primer caso en una paciente pediátrica de 14 años, que se presentó con ascitis y derrame pleural refractarios a tratamiento con elevación de CA-125, sin encontrar tumor ovárico, que ameritó manejo agresivo.
The presence of ovarian tumor (primary fibroma, thecoma, Brenner tumor), pleural effusion and ascites is referred to as Meigs’ syndrome. When it is associated with tumors other than those mentioned above, it is known as pseudo-Meigs’ syndrome. Tjalma described pseudo-pseudo-Meigs’ syndrome as a clinical condition that is accompanied by pleural effusion, ascites and elevated cancer antigen (CA) 125, with no associated benign or malignant ovarian tumor, in patients with systemic lupus erythematosus (SLE).1–4 We present the case of a 14-year-old girl with Tjalma syndrome, to date the only report of this condition in a pediatric patient.
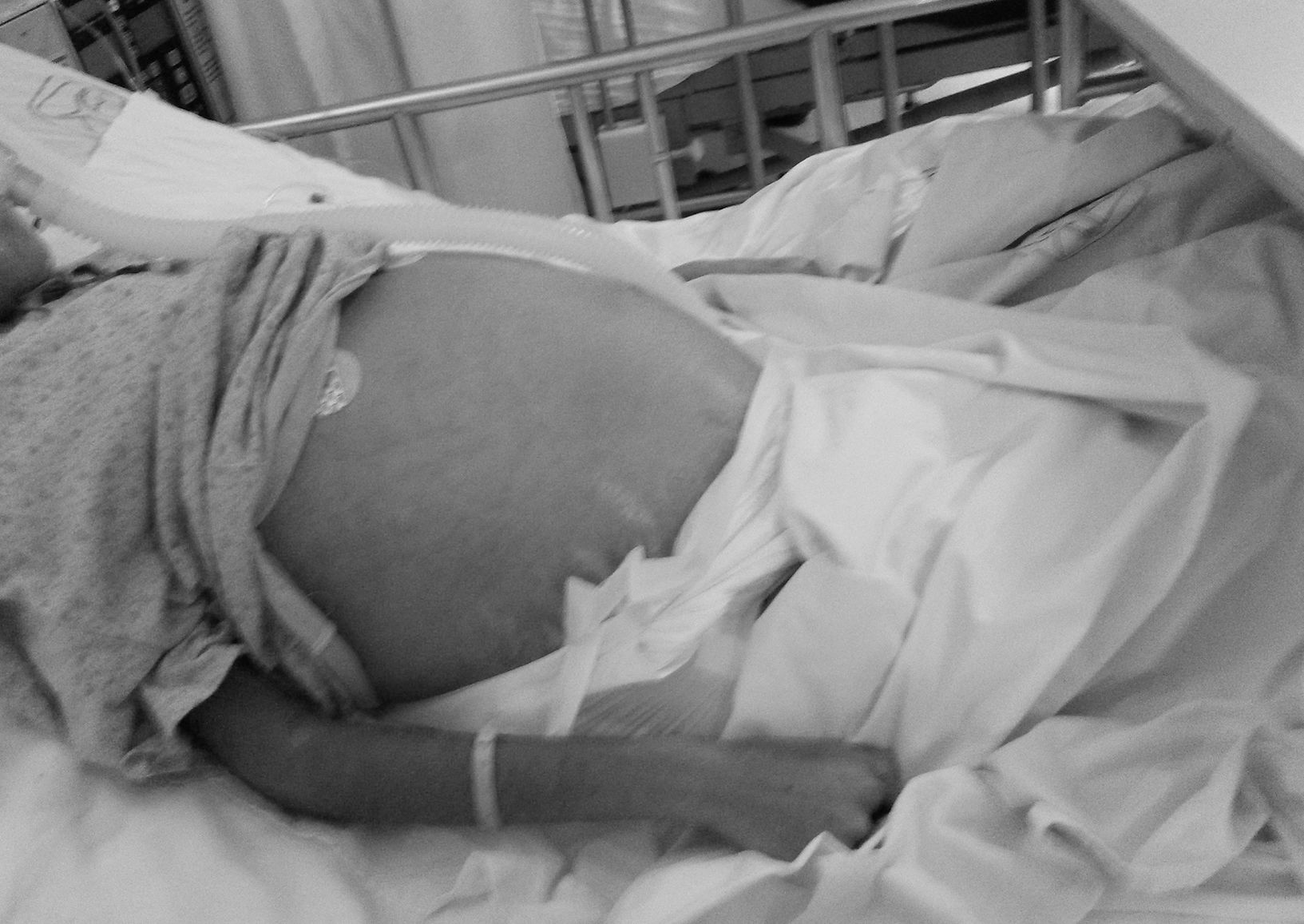
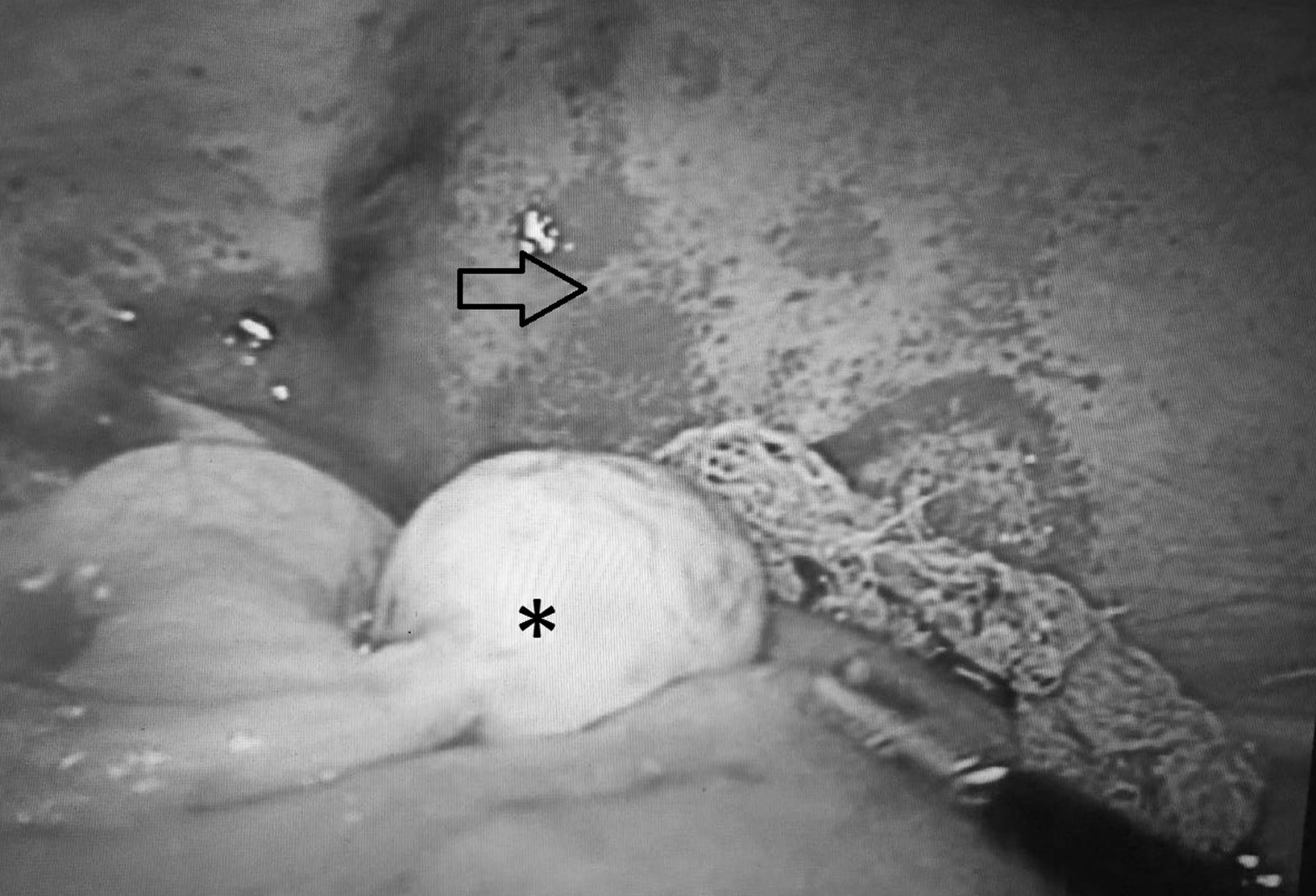
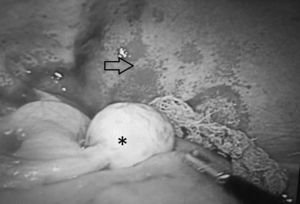
Case ReportThe patient was a 14-year-old girl who had a history of 2 weeks of edema in her lower limbs, photosensitivity, oral ulcers, cyanosis, weight loss and fever, with a diagnosis of pyelonephritis. The symptoms persisted and she came to our hospital. We found that she had arthritis, ascites, pallor and anemia of chronic disease. Her platelet count was 340,000/μL, the lymphocyte count was 870/μL, creatinine was 1.66mg/dL, urea was 164mg/dL, blood urea nitrogen was 76mg/dL, albumin was 2.14g/dL, C3 was 53mg/dL, C4 was 3.9mg/dL, antinuclear antibodies (homogeneous pattern) 1:160, anti-DNA>240, urinalysis showed red blood cells in the urine and casts; SLE was diagnosed with a Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) of 26. Treatment was initiated with methylprednisolone pulses (5 doses of 30mg/kg body weight [bw]/day), and she developed respiratory distress and left pleural effusion. She continued to have fever, anemia, acute kidney injury, thrombocytopenia, hypertension, neuropsychiatric disorders, peripheral blood smear with schistocytes, left diaphragmatic hypomotility according to thoracic ultrasound (US) and pulmonary function tests revealed a severe restrictive pattern, with a diagnosis of shrinking lung syndrome and thrombotic thrombocytopenic purpura (TTP). The treatment was plasma infusion at 30mL/kg bw/day, subcutaneous cyclophosphamide (1g/m2/month) and rituximab (total dose of 1.5g, divided into 2 doses at an interval of 14 days). Despite treatment, ascites persisted and the patient underwent paracentesis. She had acute pulmonary edema, with passage to phase III of ventilation, and cardiorespiratory arrest and acute kidney injury managed with hemodialysis. She was extubated a week later; acute kidney injury, ascites and TTP persisted. She had 5 sessions of plasmapheresis, and TTP remitted. Given refractory ascites (Fig. 1), persistent pleural effusion and US showing an increase in volume of the structures that support left ovary, we suspected Meigs’ syndrome, with a result of CA-125 elevated by 59IU/mL. Ovarian tumor was ruled out by laparotomy (Fig. 2), and we concluded that the diagnosis was Tjalma syndrome. To treat the refractory ascites, we decided to utilize an intraperitoneal steroid (dexamethasone with 2 monthly doses of 12mg). Hemodialysis was discontinued 3 months later. The patient completed the treatment with 12 monthly cycles of cyclophosphamide and gradual tapering of the steroid. She is currently taking 2g/day of mycophenolate mofetil and 5mg/day of prednisone. In the latest determination of CA-125 the level was 23IU/mL (normal value: 0–35IU/mL).
DiscussionTumor markers are utilized to detect recurrences, for the evaluation of the response to treatment or for diagnosis. CA-125 is a glycoprotein initially described by Bast in 1981. It reacted with epithelial tumor cells of ovarian cancer. Its use as a diagnostic tool is limited, since it is also expressed on the surface of cells derived from coelomic epithelium (Fallopian tubes, endometrium, endocervix, ovary, pleura, peritoneum and pericardium), lung, breast, prostate and conjunctiva, and the levels of certain cytokines like vascular endothelial growth factor (VEGF) and fibroblast growth factor increase on interacting with mesothelial cells. Their elevation is associated with other conditions, like early pregnancy, ascites, menstruation, nephrotic syndrome, endometriosis, leiomyomas, congestive heart failure, cirrhosis, SLE, rheumatoid arthritis and tuberculosis.2,5–7
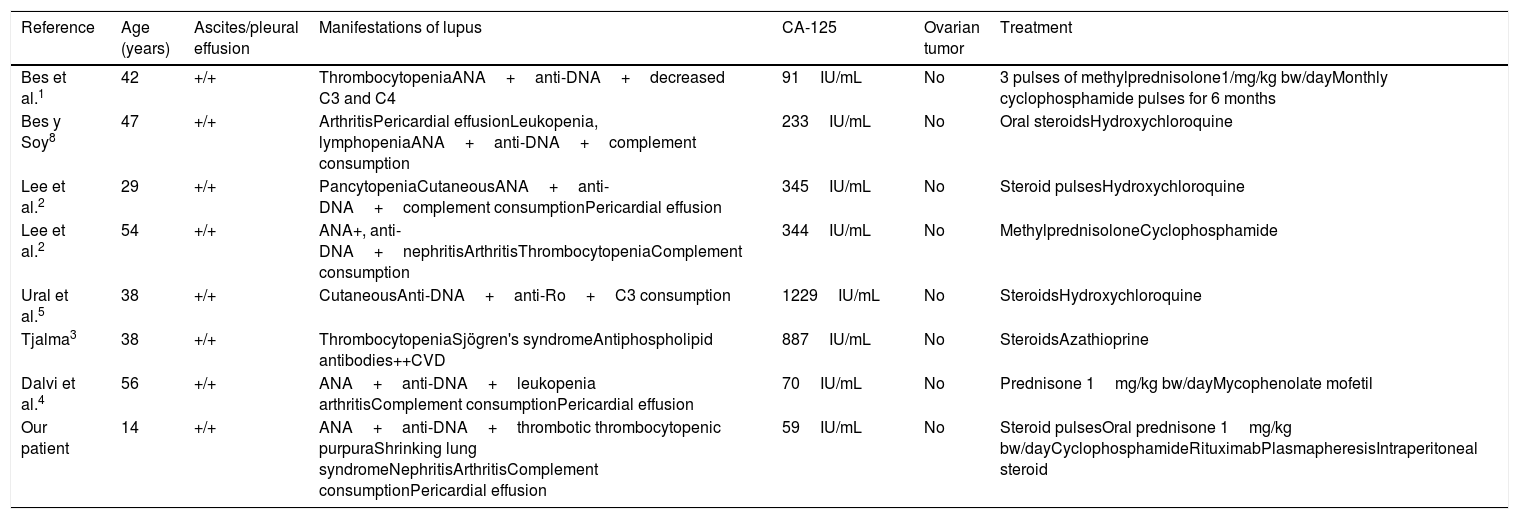
Elevated CA-125 levels in SLE are probably secondary to the activation of mesothelial cells. Cytokines like interleukin (IL) 1 and interferon (IFN) γ increase the expression of CA-125 in human peritoneal mesothelial cells.2,4 The VEGF levels are elevated in patients with nephrotic syndrome, a fact that may explain the increase in CA-125.1,8Table 1 shows the features of reported cases of Tjalma syndrome. Moncayo et al. measured the levels of CA-125 in 37 SLE patients and found elevated levels in patients with active disease.6 Szekanecz observed CA-125 elevation with disease activity, but there was no renal involvement.6 This contrasts with the findings of Miret et al.,6 who evaluated serum CA-125 levels in 59 patients with SLE and their relationship with activity. They found an elevation in 3 of them, 2 with disease activity and 1 with inactive disease, but no correlation with activity, although there was a link to nephrotic syndrome. Yang et al.6 conducted a study involving 156 SLE patients, and observed an elevation of CA-125 in 48. Compared to patients with a normal CA-125 level, those in which it was elevated were more likely to have serositis (37.5% vs 1.9%); P<.001), pulmonary involvement (37.5% vs 12%; P<.001) and a high SLEDAI score P<.007). They generated a receiver operating characteristic ROC curve, and determined the area under the curve to assess the strength of CA-125 elevation in the diagnosis of serositis due to SLE. We identified a cutoff value of 38IU/mL (sensitivity of 85%, specificity of 75%). The determination was repeated in 15 patients when the serositis resolved, and the level was normal in 14 and remained high in 1.6 Our patient had an SLEDAI score of 26, as well as uncommon and severe complications, that warranted aggressive therapy with cyclophosphamide, rituximab and plasmapheresis. For refractory peritonitis, we utilized an intraperitoneal steroid (dexamethasone at 12mg monthly in 2 doses), as reported by Yemil Atisha (Mexican Rheumatology Congress, 2015). In our case, we attributed CA-125 elevation to the presence of high disease activity, refractory serositis, congestive heart failure and nephrotic syndrome.
Characteristics of Tjalma Syndrome Patients.
| Reference | Age (years) | Ascites/pleural effusion | Manifestations of lupus | CA-125 | Ovarian tumor | Treatment |
|---|---|---|---|---|---|---|
| Bes et al.1 | 42 | +/+ | ThrombocytopeniaANA+anti-DNA+decreased C3 and C4 | 91IU/mL | No | 3 pulses of methylprednisolone1/mg/kg bw/dayMonthly cyclophosphamide pulses for 6 months |
| Bes y Soy8 | 47 | +/+ | ArthritisPericardial effusionLeukopenia, lymphopeniaANA+anti-DNA+complement consumption | 233IU/mL | No | Oral steroidsHydroxychloroquine |
| Lee et al.2 | 29 | +/+ | PancytopeniaCutaneousANA+anti-DNA+complement consumptionPericardial effusion | 345IU/mL | No | Steroid pulsesHydroxychloroquine |
| Lee et al.2 | 54 | +/+ | ANA+, anti-DNA+nephritisArthritisThrombocytopeniaComplement consumption | 344IU/mL | No | MethylprednisoloneCyclophosphamide |
| Ural et al.5 | 38 | +/+ | CutaneousAnti-DNA+anti-Ro+C3 consumption | 1229IU/mL | No | SteroidsHydroxychloroquine |
| Tjalma3 | 38 | +/+ | ThrombocytopeniaSjögren's syndromeAntiphospholipid antibodies++CVD | 887IU/mL | No | SteroidsAzathioprine |
| Dalvi et al.4 | 56 | +/+ | ANA+anti-DNA+leukopenia arthritisComplement consumptionPericardial effusion | 70IU/mL | No | Prednisone 1mg/kg bw/dayMycophenolate mofetil |
| Our patient | 14 | +/+ | ANA+anti-DNA+thrombotic thrombocytopenic purpuraShrinking lung syndromeNephritisArthritisComplement consumptionPericardial effusion | 59IU/mL | No | Steroid pulsesOral prednisone 1mg/kg bw/dayCyclophosphamideRituximabPlasmapheresisIntraperitoneal steroid |
ANA, antinuclear antibodies; bw, body weight; CA, cancer antigen; CVD, cerebrovascular disease; DNA, deoxyribonucleic acid.
Tjalma syndrome is a rare association that had not been reported until now in the pediatric age group. Although CA-125 elevation requires ruling out cancer, it is also necessary to assess other causes of the elevation of CA-125 like those mentioned above.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Torres Jiménez AR, Solís-Vallejo E, Céspedes-Cruz AI, Zeferino Cruz M, Rojas-Curiel EZ, Sánchez-Jara B. Síndrome de Tjalma (pseudo-pseudo Meigs) como manifestación inicial de lupus eritematoso sistémico de inicio juvenil. Reumatol Clin. 2019;15:e41–e43.