To analyze the Spanish experience in an international study which evaluated tocilizumab in patients with rheumatoid arthritis (RA) and an inadequate response to conventional disease-modifying antirheumatic drugs (DMARDs) or tumor necrosis factor inhibitors (TNFis) in a clinical practice setting.
Material and methodsSubanalysis of 170 patients with RA from Spain who participated in a phase IIIb, open-label, international clinical trial. Patients presented inadequate response to DMARDs or TNFis. They received 8mg/kg of tocilizumab every 4 weeks in combination with a DMARD or as monotherapy during 20 weeks. Safety and efficacy of tocilizumab were analyzed. Special emphasis was placed on differences between failure to a DMARD or to a TNFi and the need to switch to tocilizumab with or without a washout period in patients who had previously received TNFi.
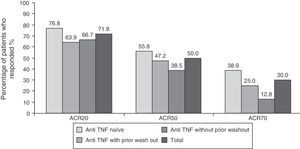
ResultsThe most common adverse events were infections (25%), increased total cholesterol (38%) and transaminases (15%). Five patients discontinued the study due to an adverse event. After six months of tocilizumab treatment, 71/50/30% of patients had ACR 20/50/70 responses, respectively. A higher proportion of TNFi-naive patients presented an ACR20 response: 76% compared to 64% in the TNFi group with previous washout and 66% in the TNFi group without previous washout.
ConclusionsSafety results were consistent with previous results in patients with RA and an inadequate response to DMARDs or TNFis. Tocilizumab is more effective in patients who did not respond to conventional DMARDs than in patients who did not respond to TNFis.
Analizar la experiencia española en un estudio internacional para evaluar tocilizumab en pacientes con artritis reumatoide (AR) con respuesta insuficiente al tratamiento con fármacos antirreumáticos modificadores de la enfermedad convencionales (FAME) o anti-TNF en condiciones cercanas a la práctica clínica habitual.
Material y métodosSubanálisis de 170 pacientes con AR que participaron en España en un ensayo clínico, internacional abierto de fase iiib, que presentaban una respuesta inadecuada al tratamiento con FAME o anti-TNF. Los pacientes recibieron 8mg/kg de tocilizumab cada 4 semanas en combinación con FAME o en monoterapia durante un periodo de 20 semanas. Se evaluaron la seguridad y la eficacia de tocilizumab distinguiendo entre pacientes con fallo a FAME o anti-TNF y, dentro de estos, entre los que habían hecho o no periodo de lavado del anti-TNF.
ResultadosLos acontecimientos adversos más frecuentes fueron infecciones (25%) y elevación de colesterol total (38%) y transaminasas (15%). Cinco pacientes abandonaron el estudio por un acontecimiento adverso. El 71/50/30% de los pacientes cumplía criterios de respuesta ACR 20/50/70 a los 6 meses del inicio del tratamiento con tocilizumab. Los pacientes naïve para anti-TNF presentaron una mayor respuesta ACR20: el 76% frente a un 64% en el grupo anti-TNF con lavado previo y el 66% en el grupo anti-TNF sin lavado previo.
ConclusionesSe confirma el perfil de seguridad de tocilizumab en pacientes con AR y fallo a FAME o anti-TNF. Tocilizumab es más eficaz en pacientes que no responden de forma satisfactoria al tratamiento con FAME convencionales que con anti-TNF.
Rheumatoid arthritis (RA) is one of the most common chronic inflammatory diseases in Western countries. The estimated prevalence of this disease in Spain is 0.5%.1 Pharmacological treatment of RA focuses on reducing inflammatory activity and preventing the progression of joint damage and its consequences. The consensus of experts from the Spanish Society of Rheumatology for RA management places full remission as therapeutic target of the disease and, in the case of patients with longstanding RA, achieving a low activity of the same.2
Disease-modifying antirheumatic drugs (DMARDs) are the treatment of choice as soon as the RA diagnosis is confirmed.2 Among the various DMARD, Methotrexate's profile of efficacy and safety justifies its use as the drug of choice for initial treatment, unless contraindicated.2,3 As a second step of treatment in patients with an insufficient response, toxicity or intolerance to conventional DMARD, biologic therapy treatment is recommended, and antagonists of tumor necrosis factor (anti-TNF) are the most frequently used drugs.2 However, between 30 and 40% of patients have an unsatisfactory response to anti-TNF, which has led to the search for new treatments for this disease.4–7
Among them, tocilizumab represents new therapeutic option. It is a monoclonal antibody that binds to the soluble and membrane bound interleukin-6 receptors, inhibiting the activity of this proinflammatory cytokine.8 Different clinical trials have demonstrated the good efficacy and safety profile of tocilizumab in different populations of patients with RA, as in the case of patients with inadequate response to conventional DMARDs or anti-TNF agents.9–12 Despite the good results observed, there are still open questions about the use of tocilizumab in routine clinical practice, such as its use in less selected patients than those included in clinical trials or the need for a washout period of prior anti TNF employed before starting treatment with this antibody.
The objective of this national study was to evaluate the safety and efficacy of tocilizumab in RA patients with a profile closer to routine clinical practice, including more comorbidities, treatments combining DMARD or absence of washout of anti-TNF.13 The high Spanish participation led us to make this subanalysis with the idea of specifically analyzing the behavior of the sample of Spanish patients.
Patients and MethodsThe open phase III b-ACT SURE clinical trial, included patients from 25 different countries.13 The results obtained in the patients from Spain are described in this paper. The study was conducted in 28 Spanish centers. The study was conducted according to the ethical principles of the Declaration of Helsinki and was approved by the Ethics Committee for Clinical Research of each unit. All patients provided informed consent before undergoing any study procedures.
The study included patients of both genders, aged 18 or over, with moderate or high RA activity (Disease Activity Score [DAS] 28>3.2) for 6 months or more, who had been treated with conventional DMARDs or anti-TNF or both types of agents on stable doses for longer than 8 weeks. Patients who were receiving glucocorticoids were receiving stable doses of ≤10mg/day of prednisone or equivalent. Those patients who had been previously treated with abatacept, anakinra or rituximab were excluded from the study. After inclusion in the study, patients received 8mg/kg of tocilizumab by intravenous infusion every 4 weeks during a period of 20 weeks (6 infusions in total). Patients could receive tocilizumab monotherapy or in combination with DMARDs, at the discretion of the investigator.
Safety was evaluated in the population of patients who had received at least one dose of tocilizumab and had at least one post-baseline safety assessment. In order to assess the need for an anti-TNF washout period before starting treatment with Tocilizumab, a subgroup analysis was planned once the database was closed. 3 groups of patients were considered: patients who had not received prior treatment with anti-TNF (anti-TNF naïve), patients who had discontinued treatment with anti-TNF at least 2 months before the first infusion of tocilizumab (anti-TNF with wash out) and a third group consisted of patients who were treated with anti-TNF and where treatment with tocilizumab was started without a wash out (anti-TNF without wash out).
Efficacy parameters proposed by the American College of Rheumatology (ACR) and DAS28 were measured at each study visit and at 4 weeks after the last infusion of tocilizumab. The efficacy of treatment with tocilizumab was assessed as the percentage of patients who met ACR20/50/70 response criteria and the percentage of patients who met the criteria for remission and low disease activity d based on DAS28 (DAS28<2, 6 and DAS28≤3.2, respectively) after 6 months of starting treatment with tocilizumab. Different parameters allowed the subsequent calculation of the Simplified Disease Activity Index (SDAI) and Clinical Disease Activity Index (CDAI) composite indexes. Additionally, the percentage of patients who met the European League Against Rheumatism (EULAR) criteria of good or moderate response at 6 months after the first infusion of tocilizumab response was estimated. The disability associated with the disease was assessed using the Health Assessment Questionnaire Disability Index (HAQ-DI). The safety of treatment with tocilizumab was assessed by monitoring adverse events and analysis of hematological and biochemical parameters in each of the study visits. Infusion reactions were considered adverse events if they occurred during the 24h following the infusion of tocilizumab. The intensity of adverse events and their relationship to the study medication were assessed by researchers.
The efficacy of treatment with tocilizumab was assessed as an intent to treat population, which consisted of patients who had received one or more doses of tocilizumab. Patients who discontinued the study prematurely were considered as non-responders in the efficacy analysis according to the ACR criteria. The analysis of the other endpoints was performed on the number of patients with available data for each of the visits. Descriptive statistics for all variables obtained were performed: mean±standard deviation (SD) for continuous variables and frequencies for categorical variables.
ResultsDemographic Characteristics and DispositionOf the 1681 patients included in the ACT-SURE international study, 170 patients belonged to the Spanish hospitals participating. The distribution of patients among the 3 groups was similar to that of the overall study: 95 were anti-TNF naïve, 36 had received anti-TNF and underwent a wash out period and 39 had received anti-TNF without wash out. The clinical and demographic baseline characteristics for all patients and different groups were considered depending on the pretreatment with anti-TNF characteristics and are presented in Table 1. Most patients in the study received tocilizumab with another DMARD; only 19 (11.2%) patients received tocilizumab monotherapy, similar to the overall study (14%) figure. 1.8% of patients received tocilizumab in combination with 2 DMARD, significantly lower than the overall study (19%). Methotrexate was the most commonly used DMARD (62.9%) at a mean dose±SD 15.9 (5.0) mg/week.15.3% of patients received leflunomide at a mean dose of 19.6±2.0mg/day. 72.4% received concomitant corticosteroids. Patients had a high baseline activity (DAS28 6.1) and duration of RA, almost 10 years, similar to the overall study data.
Baseline Demographic and Clinical Characteristics.
| Anti-TNF naïve (No.=95) | Anti-TNF with prior wash out (No.=36) | Anti-TNF without wash out (No.=39) | Total (No.=170) | |
| Age (years), mean±SD | 54.2±12.2 | 52.1±12.2 | 51.9±10.8 | 53.2±11.9 |
| Gender (women), n (%) | 84 (88.4) | 31 (86.1) | 37 (94.9) | 152 (89.4) |
| Duration of RA (years), mean±SD | 8.8±8.2 | 9.9±5.9 | 7.8±11.6 | 9.7±7.7 |
| Number of concomitant DMARDs, n (%) | ||||
| 0 | 9 (9.5) | 3 (8.3) | 7 (17.9) | 19 (11.2) |
| 1 | 84 (88.4) | 32 (88.9) | 32 (82.1) | 148 (87.1) |
| 2 | 2 (2.1) | 1 (2.8) | - | 3 (1.8) |
| Patients taking leflunomide, n (%) | 10 (10.5) | 8 (22.2) | 8 (20.5) | 26 (15.3) |
| Patients treated with methotrexate, n (%) | 63 (66.3) | 23 (63.9) | 21 (53.8) | 107 (62.9) |
| Patients treated with corticosteroids, n (%) | 59 (62.1) | 34 (94.4) | 30 (76.9) | 123 (72.4) |
| CRP (mg/dl), mean±SD | 1.3±1.9 | 2.2±2.6 | 2.2±3.1 | 1.7±2.4 |
| ESR (mm/h), mean±SD | 42.1±24.4 | 48.6±30.2 | 49.3±27.8 | 45.1±26.6 |
| NPJ, mean±SD | 19.5±13.7 | 19.2±16.2 | 22.2±14.7 | 20.1±14.5 |
| NSJ, mean±SD | 9.4±11.0 | 8.5±11.0 | 14.1±11.3 | 9.7±11.7 |
| DAS28, mean±SD | 6.0±1.2 | 6.1±1.1 | 6.3±1.1 | 6.1±1.1 |
| SDAI, mean±SD | 33.9±15.0 | 36.3±15.7 | 38.6±14.7 | 35.5±15.1 |
| CDAI, mean±SD | 32.6±14.2 | 34.1±15.1 | 36.4±14.4 | 33.8±14.4 |
RA: rheumatoid arthritis; CDAI: Clinical Disease Activity Index; DAS28: Disease Activity Score based on 28 joints; SD: standard deviation; DMARD: disease-modifying antirheumatic drugs; NPJ: number of painful joints; NSJ: number of swollen joints; CRP: C Reactive Protein; SDAI: Simplified Disease Activity Index; ESR: erythrocyte sedimentation rate.
88.8% (n=151) of patients completed the 6-month study, similar to the overall study. The reasons for study discontinuation were withdrawal of consent in 5 patients, adverse event in 5 patients, inadequate treatment response in 3 patients, investigator's decision in 2 patients, violation of any selection criteria in 2 patients and loss to follow in 2 patients.
SafetyThe main results of the safety study are presented in Table 2. Overall, adverse events were recorded in 118 patients. Those who had previously been treated with anti-TNF, and the had undergone a prior wash out, showed a lower apparent numerical incidence of adverse events, unlike the global study, in which no safety differences were detected in terms of unwashed or washed out patients. Infections were one of the most common adverse events, 25.9% of patients had at least one infection during the study. A single patient had an upper respiratory tract infection that was considered a serious adverse event (1.1 per 100 patient-years), compared with 5.1 per 100 patient-years in the overall study. 15.3% of patients experienced infusion reactions, defined as any adverse event occurring during or within the following 24h infusion, this figure was numerically lower in the group of washed out patients (8, 3%). No deaths were recorded during the study. Non-infectious adverse events leading to withdrawal from the study were: humerus fracture, ulcerative keratitis, laryngeal cancer and respiratory failure.
Results Regarding Safety.
| Patients | Anti-TNF naïve (No.=95) | Anti-TNF with prior wash out (No.=36) | Anti-TNF with prior wash out (No.=39) | Total (No.=170) |
| AE, n (%) | 69 (72.6) | 21 (58.3) | 28 (71.8) | 118 (69.4) |
| SAE, n (%) | 5 (5.3) | 2 (5.6) | 1 (2.6) | 8 (4.7) |
| Infections, n (%) | 28 (29.5) | 6 (16.7) | 10 (25.6) | 44 (25.9) |
| Serious infections, n (%) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Infusion reactions, n (%) | 17 (17.9) | 3 (8.3) | 6 (15.4) | 26 (15.3) |
| ALT elevation<1.5×ULN, n (%) | 19 (20.0) | 3 (8.3) | 6 (15.3) | 28 (16.4) |
| Elevation cholesterol values>200mg/dl, n (%) | 40 (42.1) | 13 (36.1) | 12 (30.8) | 65 (38.2) |
| Neutrophil count<1500/mm3, n (%) | 16 (16.8) | 4 (11.1) | 5 (12.8) | 25 (14.7) |
AE: adverse events; SAE: serious adverse events, ALT: alanine aminotransferase, ULN: upper limit of normal.
14.7% of patients developed neutropenia during treatment with tocilizumab, in no cases was the neutrophil count less than 500/mm3. 16.4% of patients had elevated levels of alanine aminostransferase (ALT), this being more frequent in the anti-TNF naïve population; only 1.7% of patients had elevated ALT between 3 and 5 times the upper limit of normal, with no case exceeding this figure. 3 8.2% of patients had elevations in total cholesterol.
EfficacyEfficacy data were in line with those of the overall study. 71.8% (n=122), 50% (n=85) and 30% (n=51) of patients respectively met ACR20, ACR50 and ACR70 response criteria at 6 months after initiation of treatment with tocilizumab (Fig. 1). The high percentage of patients achieving ACR20 response after receiving the first infusion of tocilizumab (44.7%) is noteworthy. This percentage increased progressively until week 16, remaining roughly constant until the visit at 6 months (data not shown). The ACR20 response at 6 months in the naïve to anti-TNF group was higher than in the other two groups (76.8% vs 63.9% in the anti-TNF group with prior wash out and 66.7% in the anti-TNF without wash out), in accordance to what has been observed in other studies with tocilizumab.9,12
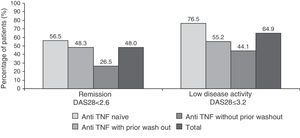
48 and 64.9% of patients had clinical remission and low disease activity, respectively, according to the DAS28 index after 6 months of treatment (Fig. 2. These responses were also higher in patients naïve to anti-TNF (76.5% vs 55.2% in the anti-TNF group with prior wash out and 44.1% in the anti-TNF group without wash out).
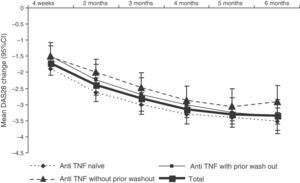
DAS28 activity decreased progressively during treatment with tocilizumab (Fig. 3. The mean DAS28 at 6 months of treatment was 2.7±1.3, representing a mean improvement of 3.4±1.5 points in all patients.
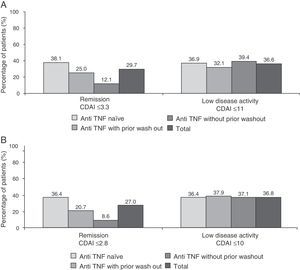
Fig. 4 shows the percentage of patients in clinical remission or low disease activity at 6 months of treatment as scored by the SDAI and CDAI criteria. As can be seen, the response to these 2 scores (the only difference is that the CDAI does not include acute phase reactants) were very similar in different subgroups of patients. As to the EULAR response, 84.1% of patients had a good or moderate response at 6 months (86.3% of anti-TNF naïve patients, 80.5% of the anti-TNF group with wash out and 82.1% of those receiving prior anti-TNF without wash out). Additionally, the majority of patients (75.5%) showed clinically significant improvement in disability questionnaire HAQ-DI (reduction of 0.22 points or more) (data not shown).
This study included patients treated with tocilizumab, both as monotherapy and in combination with DMARDs. The ACR20, 50 and 70 responses showed no significant differences between treatment groups (data not shown)
DiscussionThe added value of the ACT-SURE study lies in its design, with a profile of RA patients similar to what is seen in routine clinical practice, including populations with broader comorbidities, patients with refractory to treatment (failure to anti-TNF and DMARD) and who could receive tocilizumab as monotherapy or in combination with different DMARD at doses near the maximum used, including combinations thereof. In addition, it had the option to perform, or not, a washout period of anti-TNF before starting tocilizumab.
This design is particularly important when analyzing safety data. The overall results of ACT-SURE have confirmed the safety profile of tocilizumab observed in previous studies.9–12 The incidence of adverse events in the subgroup of patients in Spain is similar to that reported in the overall study.13 The main adverse effects detected were infections and altered laboratory parameters. However, some differences are observed between both studies. It is noteworthy that in the Spanish subpopulation there was only one case of serious infection among 170 patients enrolled (1.1 per 100 patient-years), compared to a rate of 5.1 per 100 patient-years in the overall study. Another safety related issue was that, in the analysis of our population, infusion reactions, infections and transaminase elevations occurred less frequently in patients with anti-TNF wash out than in the rest.
The reasons for these discrepancies are unknown. In principle, it does not seem attributable to differences in the patient profile, given that the distribution between the 3 subgroups, and the time since onset of RA or the baseline activity were similar in the 2 analyses. The lower frequency of patients treated with the combination of tocilizumab and 2 or more DMARDs in our population could be a differentiating factor, but we do not know if this was associated with a higher rate of serious infections in the global analysis.13 The fact that the number of Spanish patients in the subgroup who underwent an anti-TNF wash out is only 36 (21%) makes it likely to be only a random variation. This is important because the overall analysis suggests that it is not necessary to perform a wash of anti-TNF before treatment with tocilizumab, which has important practical implications.
In terms of efficiency, our data confirm the general result of tocilizumab, with a fast and consistent effect both in patients naïve to anti-TNF as in those with an inadequate response to these drugs. As expected, the response is better in the first group of patients than in the second. This coincides with the results previously observed with tocilizumab.9–12 Minor differences in tocilizumab efficacy after performing anti-TNF wash out are likely related to the limited number of patients with baseline differences in the activity and duration of illness. The patients in whom prior wash out was not performed had a more severe and longer duration than patients in those whom this wash out was performed. In the overall analysis of the data, there were no differences in efficacy between these groups.13
The highest percentage of responders according to the DAS28 index compared with the SDAI and CDAI can be explained by the significant effect tocilizumab has on acute phase reactants and the weight that the ESR has in the DAS28 formula. However, the fact that SDAI and CDAI responses (2 indices which differ only in that CDAI does not include acute phase reactants) are similar shows that the efficacy of tocilizumab is not primarily due to its effect on reactants.
It is interesting to note that the efficacy of tocilizumab monotherapy in this analysis is similar to that of its combined use with DMARD. While the fact that patients were not randomized based on this parameter limits the significance of this data, other results support this idea,14 which makes tocilizumab a good choice for patients intolerant to or with contraindications to DMARD. Furthermore, a recent study has demonstrated the superiority of tocilizumab versus adalimumab monotherapy.15
The main limitations of this analysis lie in the number of patients and duration of the 6-month follow-up. Although the analysis of longer periods of treatment is essential to evaluate the safety of a drug, our results are consistent with other studies of longer duration.16 In contrast, the profile of less selected patients and, therefore, closer to actual clinical practice, provides great value to this data.
In conclusion, the results of this study allow us to confirm the safety profile of tocilizumab in RA patients and failure to DMARD or anti-TNF. Tocilizumab appears to be more effective in patients who do not respond satisfactorily to treatment with conventional DMARDs than those not responding to anti-TNF.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that study procedures conformed to the ethical standards of the responsible committee on human experimentation and in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentialityThe authors declare that they have followed the protocols of their workplace regarding the publication of data from patients and that all patients included in the study have received sufficient information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained informed consent from patients and/or subjects referred to in the article. This document is in the possession of the corresponding author.
Conflict of InterestRoche Pharma has been the ACT SURE study sponsor. J.M. Alvaro-Gracia has received research support or honoraria for consultancy or presentations from Abbott, Aventis, Bristol Myers Squibb, Janssen-Cilag, MSD, Pfizer, Roche, and UCB Tigenix; FJ White has received research support or honoraria from Absciex, Abbott, Ardea Bioscience, Bioiberica, Bristol Myers Squibb, Celgene, Celltrion, Lilly, Merck, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi Aventis, and UCB Tigenix; A. Fernández-Nebro has received research support or honoraria for lectures from Abbott, MSD, Pfizer and Roche, A. Garcia-Lopez has received research support or honoraria from Abbott, Amgen, Bristol Myers Squibb, MSD, Roche and UCB; FJ Navarro has received research support or honoraria from Abbott, Celltrion, Roche and UCB; S. Bustabad has received research grants and honoraria from Roche, MSD, UCB, Abbott and Bristol Myers Squibb; Y. Armendariz is employed by the Medical Department of Roche, and JA Román-Ivorra has received research support or honoraria from Abbott, Actelion, Amgen, Bristol Myers Squibb, Merck, Pfizer, Roche and UCB.
The manuscript authors wish to thank the participation of researchers in the ACT SURE study as well as for the support provided by Marta Muñoz Tudurí, Unit of Medical Writing of TFS Develop Spain.
Jose Luis Alvarez Vega
Jose Maria Alvaro-Gracia
Arboleya Luis Rodríguez
Joaquín Belzunegui Otano
Francisco Blanco García
M. Sagrario Bustabad Reyes
Javier Calvo Catala
Cesar Diaz Torne
Antonio Domingo Gómez Centeno
Escudero Alejandro Contreras
Luis Fernández Domínguez
Antonio Fernández Nebro
Angel Aparicio García
Sergio García Pérez
Alicia García Testal
Jose Garcia Torón
Eduardo Quesada Girona
Guzmán Manuel Ubeda
Ibero Isabel Diaz
Francisco Javier Ruiz Manero
Carlos Fernandez-Cid Marras
Sara Marsal Barrel
Francisco Javier Navarro Blasco
Francisco Javier García Narváez
Lucia Pantoja Zarza
María Trinidad Pérez Sandoval
Javier del Pino Montes
Paula Ramos Carmen Nunez
Elena Riera Alonso
Diaz Manuel Riesco
Jose Ramon Rodriguez Cros
Jose Manuel Rodriguez Heredia
Carlos Rodríguez Lozano
José Andrés Román Ivorra
Rosello Rosa Pardo
Jose Javier Salaberri Maestrojuan
Raimon Sanmarti Room
Manuel Utrilla Utrilla
Tomás Vázquez Ramón Rodríguez
Paloma Vela Casasempere
Please cite this article as: Álvaro-Gracia JM, Fernández-Nebro A, García-López A, Guzmán M, Blanco FJ, Navarro FJ, et al. Tocilizumab en pacientes con artritis reumatoide activa y respuesta inadecuada a fármacos antirreumáticos modificadores de la enfermedad o antagonistas del factor de necrosis tumoral: subanálisis de los datos españoles de un estudio abierto cercano a la práctica clínica habitual. Reumatol Clin. 2014;10:94–100.