Ankylosing spondylitis (AS) is a chronic inflammatory disease characterized by sacroiliac inflammation and inflammatory low back pain. It belongs to the spondyloarthritis group of disease, which has the common denominator of the presence of sacroiliitis, extra-articular manifestations and HLA-B27 positivity.1,2
Conventional treatment with disease modifying antirheumatic drugs (DMARDs) has limited efficacy, particularly in patients with axial involvement, due to which the use of biologic therapy with monoclonal antibodies against tumor necrosis factor (anti-TNF), including adalimumab, was introduced and which has led to3 improved clinical responses. Among the adverse events of anti-TNF drugs, there are reported cases of elevated liver enzymes, aspartate aminotransferase (AST) and alanine aminotransferase (ALT),4 and even5 subacute liver failure.
We report the case of a 32-year-old man with AS of 2 years evolution, HLA-B27 positive, with a poor response to sulfasalazine 1g/8h, and NSAIDsl, who had persistent severe pain in the lumbosacral region associated with stiffness and functional limitation. Physical examination revealed pain on the sacroiliac joints and arc movement limitation. MRI evidenced spinal osteitis, spinal cord edema and early changes of sacroiliac ankylosis.
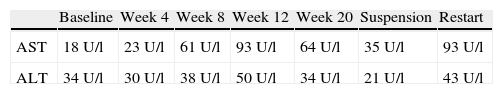
AS was considered as in progression, with a high score on the BASFI and BASDAI scales, for which treatment with adalimumab was initiated at a dose of 40mg every 15 days, achieving an adequate clinical response. During follow-up, progressive elevation of aminotransferases was documented, with bilirubin and alkaline phosphatase within normal limits. Since at that time the patient had received no other medication, possible hepatotoxicity adalimumab was suspected, so the biological therapy (Table 1) was suspended with a decline in the aminotransferase levels. A diagnostic test was done with the administration of another dose of adalimumab, once aminotransferases normalized, with a new elevation thereof seen, confirming the case as drug-induced. The diagnostic approach was complemented with tests for viral B and C hepatitis virus, anti-smooth muscle, antimitochondrial antibodies and liver biopsy, ruling out an autoimmune origin.
Anti-TNF therapy may cause hepatotoxicity, which can range from alterations in liver function tests to cases of severe liver failure, through reactivation of viral hepatitis.6 Hagel et al. published a case of a patient aged 44 with a history of psoriasis without liver disease, who developed subacute liver failure 4 months after treatment with adalimumab. After discontinuation of therapy and initiation of prednisone, a decrease in aminotransferase levels to normal was documented. The same authors reported mild elevation of aminotransferases, up to 3 times the reference value in 1%–4% of patients treated.5 Van der Heijde et al., in 208 AS patients treated with adalimumab, reported at week 12 of follow-up, elevated aminotransferases in 6 patients, with ALT levels 3 times above the reference value, and subsequent normalization of levels in 4 of them without suspension. At 24-weeks of follow-up, only 6 patients (2.8%) had serious adverse events, including one case of elevated liver enzymes in need of liver biopsy in a patient with moderate alcohol consumption and concomitant treatment with indomethacin.7
A Japanese study documented hepatic adverse event in 31.7% of patients treated with adalimumab, including elevated aminotransferases, up to 2.5 times normal, and hepatic steatosis. In neither case was it considered a serious episode and did not require discontinuation of the drug. Cases of hepatitis B reactivation beginning with elevated aminotransferases have also been reported.2,8
Researchers of the CORRONA (Consortium of Rheumatology Researchers of North America) data collection program compared patients receiving anti-TNF therapy (infliximab, etanercept or adalimumab) and who had alterations in liver function tests, and found the following odds ratios for an increase of >2 times in liver function tests: infliximab 2.4 (95% CI: 1.53–3.76), adalimumab 1.72 (95% CI: 0.99–3.01) and etanercept 1.1 (95% CI: 0.64–1.88); however, they noted that the frequency of this disorder is rare.4
Our case presented elevated aminotransferases after initiation of adalimumab therapy, which resolved following discontinuation of the drug.
The elevation of aminotransferases is an effect that can occur in patients with AS receiving anti-TNF treatment, however, its progression to severe hepatitis is rare and in most patients is a temporary adverse event that resolves spontaneously and does not produce symptoms.
Conflict of InterestThe authors have no conflicts of interest.
Please cite this article as: Juan-Guardela ML, Marín-Carrillo LF, Kattah-Martínez LX, Fernández-Ávila DG. Elevación de transaminasas secundaria a uso de adalimumab. Reumatol Clin. 2014;10:265–266.