Tropical alphaviruses have special tropism for bone and joint tissue. Patients can develop chronic rheumatic disorders similar to rheumatoid arthritis and ankylosing spondylitis. The prototype is Chikungunya virus, although other lesser known viruses in our environment such as Sindbis, Ross River, Mayaro, O’nyong nyong and Barmah Forest viruses have the potential to be sped through vectors and cause chronic rheumatic disease.
International population movements have increased the numbers of patients diagnosed with these tropical viruses in areas in which they are not endemic. Since they can leave persistent symptoms and affect the quality of life of the patients, it is important that we be aware of them. Changes in ecosystems have favored the expansion of competent mosquitoes, making fears of local transmission in southern Europe a reality.
The objective of this review is to provide a clinical approach to the different arthritogenic tropical alphaviruses, especially those in which chronic rheumatic disease is more frequent.
Los alfavirus tropicales tienen especial tropismo por el tejido osteoarticular. Los pacientes desarrollan cuadros crónicos reumatológicos similares a la artritis reumatoide y la espondilitis anquilosante. El prototipo es el virus Chikungunya, aunque otros virus menos conocidos en nuestro medio como Sindbis, Ross River, Mayaro, O’nyong nyong y Barmah Forest tienen un potencial para propagarse a través de vectores y causar cuadros reumatológicos crónicos.
Los movimientos poblacionales internacionales han aumentado el número de pacientes diagnosticados por estos virus tropicales en zonas no endémicas. Dado que pueden dejar secuelas y afectar la calidad de vida, es importante conocerlos. Los cambios en los ecosistemas han favorecido la expansión de mosquitos competentes, haciendo realidad el temor de transmisión local en el sur de Europa.
El objetivo de esta revisión es dar una aproximación clínica de los distintos alfavirus tropicales artritogénicos, especialmente de aquellos en los que la patología reumática crónica es más frecuente.
Although there is little data on the prevalence of rheumatic diseases in tropical regions, it seems that the number of cases of rheumatoid arthritis (RA), spondyloarthritis and connective tissue diseases is increasing worldwide.1
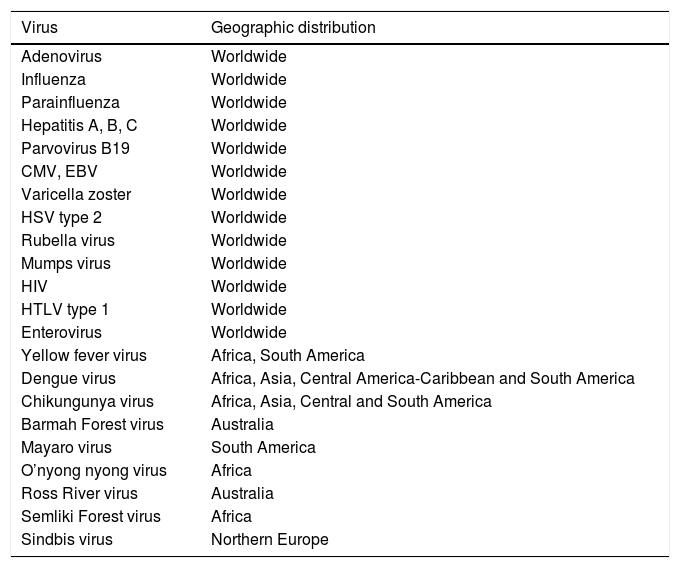
Infectious diseases are an important cause of the involvement of bone and joint tissue. Table 1 shows the viruses responsible for these manifestations and their geographic distribution.
Viruses That Cause Musculoskeletal Manifestations and Their Geographic Distribution.
| Virus | Geographic distribution |
|---|---|
| Adenovirus | Worldwide |
| Influenza | Worldwide |
| Parainfluenza | Worldwide |
| Hepatitis A, B, C | Worldwide |
| Parvovirus B19 | Worldwide |
| CMV, EBV | Worldwide |
| Varicella zoster | Worldwide |
| HSV type 2 | Worldwide |
| Rubella virus | Worldwide |
| Mumps virus | Worldwide |
| HIV | Worldwide |
| HTLV type 1 | Worldwide |
| Enterovirus | Worldwide |
| Yellow fever virus | Africa, South America |
| Dengue virus | Africa, Asia, Central America-Caribbean and South America |
| Chikungunya virus | Africa, Asia, Central and South America |
| Barmah Forest virus | Australia |
| Mayaro virus | South America |
| O’nyong nyong virus | Africa |
| Ross River virus | Australia |
| Semliki Forest virus | Africa |
| Sindbis virus | Northern Europe |
CMV, cytomegalovirus; EBV, Epstein Barr virus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; HTLV, human T lymphotropic virus.
Parvovirus B19, the rubella virus, hepatitis A virus and certain arboviruses often result in joint involvement, whereas respiratory viruses, enteroviruses, herpes family viruses and mumps virus are rarely the cause of arthritis. Arthralgia is common in other infections produced by tropical flaviviruses such as dengue virus or Zika virus. During the acute febrile phase in Zika virus, up to 65% of the patients have arthralgia or arthritis and, occasionally, swelling of hands and ankles. These manifestations occur during a short period of time and the severity is generally limited. There are a number of bacteria and fungi capable of producing reactive arthritis, which can even be severe and prolonged.2
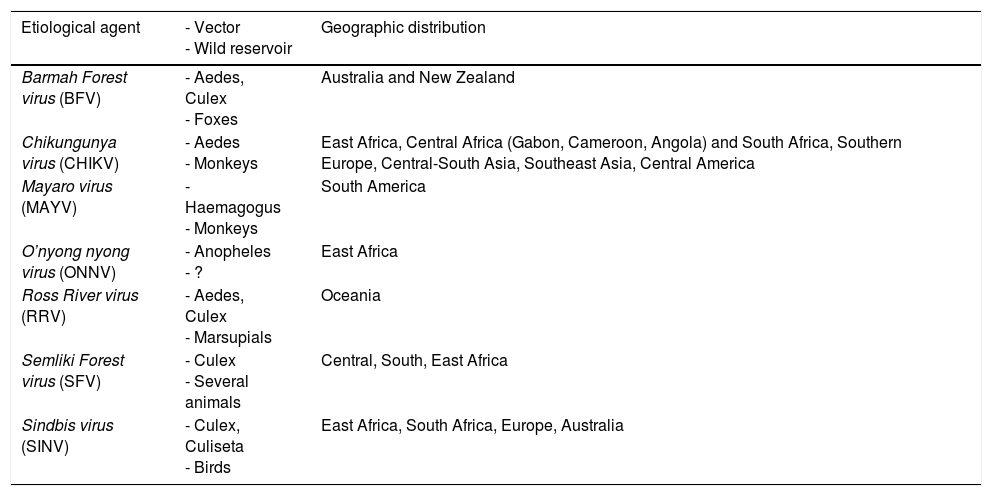
Tropical alphaviruses have a special tropism for musculoskeletal tissue. They are arboviruses that belong to the Togaviridae family, and are classified among those associated with New World meningoencephalitis and those associated with polyarthritis (Table 2). The reservoirs of these viruses are generally wild animals and, although a human can become a reservoir, mosquitos are responsible for their transmission and nonvectorial transmission from one person to another is unusual.3
Vector, Reservoir and Geographic Distribution of Alphaviruses.
| Etiological agent | - Vector - Wild reservoir | Geographic distribution |
|---|---|---|
| Barmah Forest virus (BFV) | - Aedes, Culex - Foxes | Australia and New Zealand |
| Chikungunya virus (CHIKV) | - Aedes - Monkeys | East Africa, Central Africa (Gabon, Cameroon, Angola) and South Africa, Southern Europe, Central-South Asia, Southeast Asia, Central America |
| Mayaro virus (MAYV) | - Haemagogus - Monkeys | South America |
| O’nyong nyong virus (ONNV) | - Anopheles - ? | East Africa |
| Ross River virus (RRV) | - Aedes, Culex - Marsupials | Oceania |
| Semliki Forest virus (SFV) | - Culex - Several animals | Central, South, East Africa |
| Sindbis virus (SINV) | - Culex, Culiseta - Birds | East Africa, South Africa, Europe, Australia |
The confluence of a number of factors in recent years has enabled the spread of several tropical viruses that had previously been confined to certain specific geographic regions.3 The main causes may have been the growth in the population and the increase in international travel, global trade and climate change. We are facing a globalization of tropical diseases, especially those transmitted by mosquitos and, as paradigmatic examples, we have dengue and Chikungunya virus (CHIKV) and, recently, Zika virus. Between 2009 and November 2014, the Spanish network for the study of infections imported by way of travelers and immigrants received reports on 136 cases of imported arboviruses. By July 2015, the number had increased to 228 cases, led by dengue virus, followed by CHIKV.4
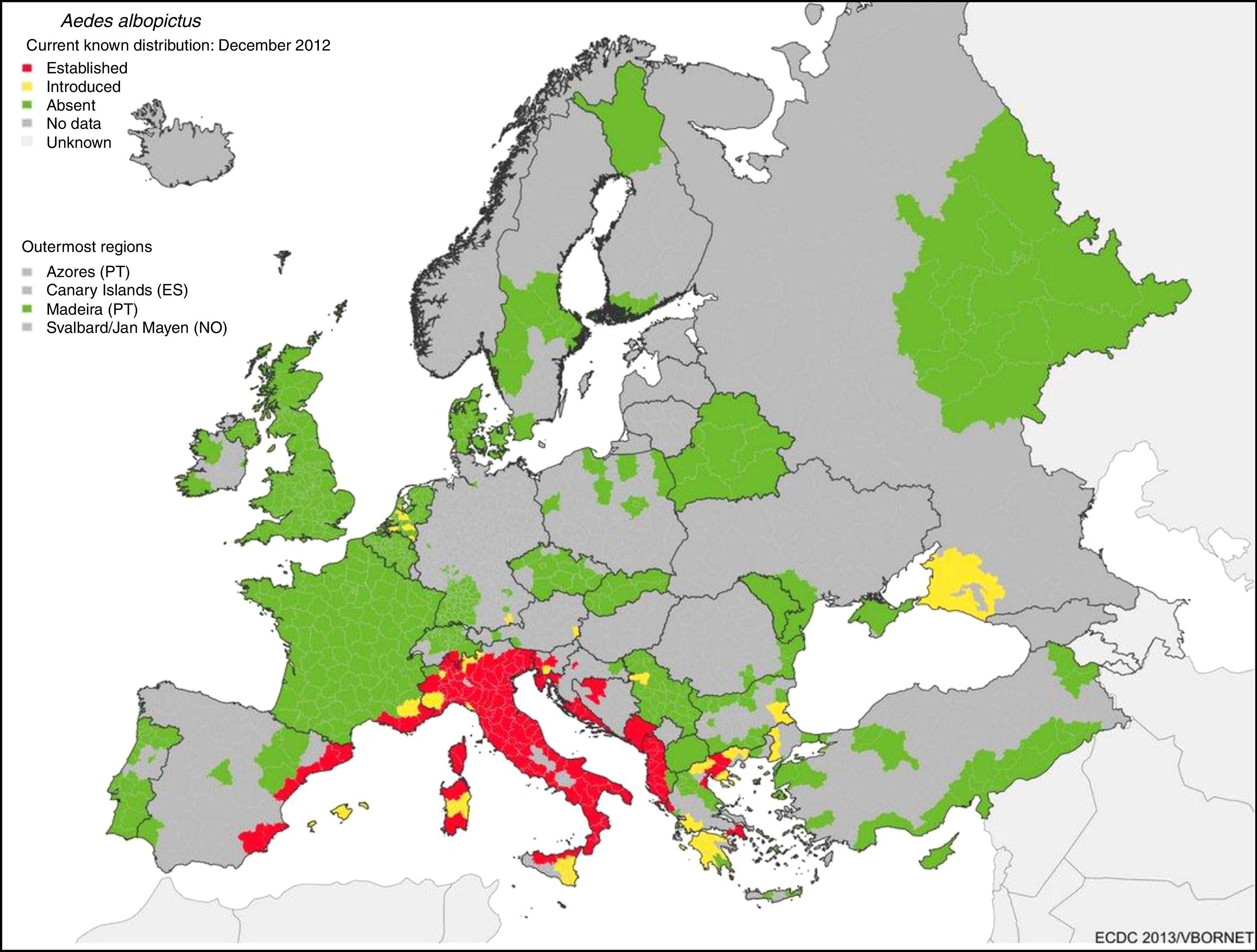
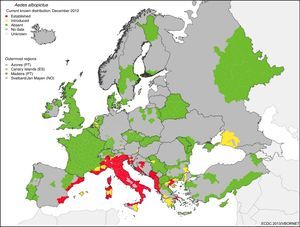
The great potential for the spread of these diseases from their tropical niche to temperate regions lies in the fact that there are competent alternative vectors widely distributed all over the world. Aedes albopictus was detected in Europe (Albania) in 1979 and in Spain (San Cugat del Vallés in Catalonia, in the northeast of the country) in 2004. Since then, it has expanded throughout southern Europe. Fig. 1 shows the risk of transmission due to the establishment of A. albopictus in temperate areas such as southern Europe.
The clinical manifestations provoked by these viral agents can, in some cases, be confused with classical rheumatic diseases because of their prolonged, fluctuating and disabling course. The objective of this review is to offer a clinical approach to the different tropical arthritogenic alphaviruses, especially those that are most often associated with chronic rheumatic manifestations.
PathophysiologyAfter inoculation via the vector bite, the virus is hematogenously disseminated through infected monocytes toward the spleen, the lymphatic system and the liver. In contrast to other viruses, they infect bone, skeletal muscle, myotendinous insertions and joint capsules. It was recently observed that Ross River virus (RRV) can affect osteoblasts, producing bone resorption, and that Sindbis virus (SINV) is capable of replication in the periosteum and the tendons of the long bones. Dissemination to the central nervous system (choroid plexuses, meninges, ependymal cells) has been observed in animals, but it is not known whether it infects neurons or brain endothelium. It does not infect trophoblasts, a fact that explains that vertical transmission occurs only during delivery. The infected target tissues undergo an extensive infiltration of lymphocytes, natural killer cells, neutrophils and macrophages.5 The pathogenesis of chronic disease is due to the conjunction of an autoimmune response to persistent viral antigens and to the chronic presence of the virus (or of its products) in the target cells, with the resulting local accumulation of inflammatory mediators.6 The virus replicates and persists in the macrophages, even if the viral burden is undetectable in peripheral blood. In macaques, that were experimentally infected with CHIKV, the presence of viral antigens and viral RNA was observed in lymphoid organs and synovial tissue months after the acute phase of the infection.7–9 In patients with chronic symptoms, biopsies in muscle and synovial tissue have shown viral RNA and antigens from RRV, CHIKV and SINV up to 6 months after the acute infection. The presence of proinflammatory cytokines is associated with the presence of arthritis or myositis. The interaction of peptides from the alphavirus capsid with human leukocyte antigen (HLA) DR4 and HLA-DR1 can cause arthritis. Interleukin 6, monocyte chemoattractant protein 1, matrix metalloproteinase 2, granulocyte-macrophage colony-stimulating factor and monokine induced gamma interferon are encountered in persistent CHIKV infection. In animal models, treatment with antibodies or inhibitors against some of these molecules and drugs that eliminate macrophages decrease the inflammatory response, with evidence of lesions that are less severe.10,11
Some patients who had previously been asymptomatic will develop manifestations of a chronic rheumatic disease, weeks or months later, that will meet the criteria for RA or ankylosing spondylitis.12 It has been demonstrated that there are peptides from the alphavirus capsid that can interact with HLA-DR4 and HLA-DR1, in such a way that the virus, together with other factors, would act as a trigger for RA.13 A number of factors associated with chronicity have been taken into account. They include female sex, age of 45 years or more, a numerical score of pain during the acute phase as being 7 or greater, symmetrical distribution at the onset of the disease and previous osteoarthritis.14,15 This provides further evidence that alphaviruses are authentic triggers for rheumatic disease.
Chikungunya VirusEpidemiologyEtymologically, the term “chikungunya” comes from Makonde (a dialect spoken by an ethnic group from southeast Tanzania and northern Mozambique) and signifies “the man who walks with a stoop”, because of the aspect of patients as a consequence of joint pain.
CHIKV is an alphavirus transmitted by mosquitos of the genus Aedes (A. albopictus and A. aegypti). The peaks of maximum activity of these vectors are during the day, especially early in the morning and at dusk.
Utilizing the gene sequencing of the protein of the viral E envelope, 3 separate lineages of CHIKV have been identified, which were detected nearly simultaneously: the Asian lineage, the West African (WA) lineage and the lineage of strains from East, Central and South Africa (ECSA).16 The viral genotype that began to affect the Indian Ocean islands and India (Indian Ocean lineage [IOL]) in 2004 is different from those that had been characterized earlier and, according to phylogenetic analyses, comes from the ECSA lineage, which has displaced the autochthonous Asian lineage.17
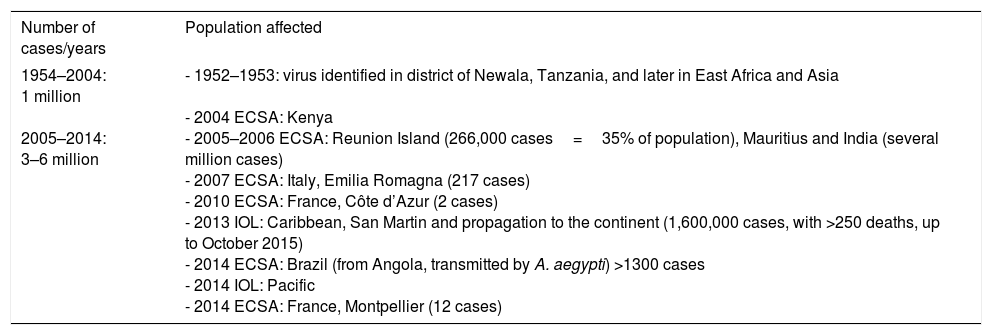
During the outbreak on Reunion Island, the E1 mutation was identified in the gene of the viral envelope (alanine-to-valine substitution in position 226 of the surface glycoprotein of ECSA-CHIKV) that enabled the virus to adapt satisfactorily to A. albopictus, enhancing its infectivity, replication and transmission.18 Likewise, transovarian transmission was also documented.19 As A. albopictus is a mosquito that survives in urban and subtropical environments, that meant that human–mosquito–human transmission became extended throughout the world. Table 3 shows the worldwide evolution of the epidemiology of CHIKV and Table 4, the cases imported in Europe and Spain.
Evolution of the Epidemiology of Chikungunya Virus.
| Number of cases/years | Population affected |
|---|---|
| 1954–2004: 1 million 2005–2014: 3–6 million | - 1952–1953: virus identified in district of Newala, Tanzania, and later in East Africa and Asia - 2004 ECSA: Kenya - 2005–2006 ECSA: Reunion Island (266,000 cases=35% of population), Mauritius and India (several million cases) - 2007 ECSA: Italy, Emilia Romagna (217 cases) - 2010 ECSA: France, Côte d’Azur (2 cases) - 2013 IOL: Caribbean, San Martin and propagation to the continent (1,600,000 cases, with >250 deaths, up to October 2015) - 2014 ECSA: Brazil (from Angola, transmitted by A. aegypti) >1300 cases - 2014 IOL: Pacific - 2014 ECSA: France, Montpellier (12 cases) |
ECSA, East-Central-South Africa; IOL, Indian Ocean “Asian” linage.
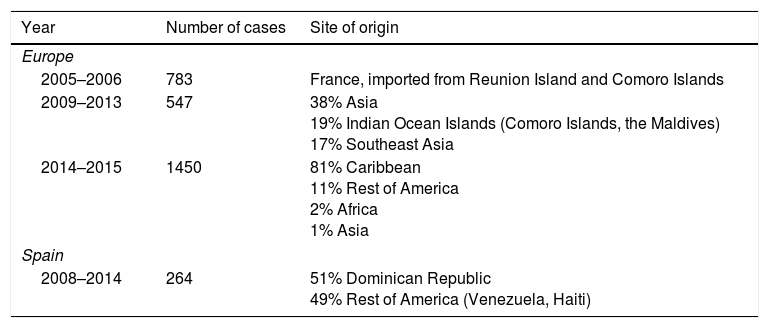
Cases of Chikungunya Virus Imported to Europe and Spain.
| Year | Number of cases | Site of origin |
|---|---|---|
| Europe | ||
| 2005–2006 | 783 | France, imported from Reunion Island and Comoro Islands |
| 2009–2013 | 547 | 38% Asia 19% Indian Ocean Islands (Comoro Islands, the Maldives) 17% Southeast Asia |
| 2014–2015 | 1450 | 81% Caribbean 11% Rest of America 2% Africa 1% Asia |
| Spain | ||
| 2008–2014 | 264 | 51% Dominican Republic 49% Rest of America (Venezuela, Haiti) |
Other documented forms of nonvectorial transmission are vertical (50% in pregnant patients who are viremic at the time of delivery) and in corneal transplantation. Blood-borne transmission has not been documented but is considered to be possible.
Individuals who have been exposed to CHIKV develop prolonged immunity that protects them from reinfection.
Prior to 2004, there were few studies on CHIKV, probably because it was considered a benign disease and was not given much importance. However, the outbreak in the Indian Ocean Islands (2004–2011), in which up to 50% of the population was reportedly affected, left individuals with severe and chronic disability. Since then, a number of publications have dealt with the outcome of the rheumatic manifestations observed in the patients who were infected in earlier epidemics, making it possible to evaluate the prognosis of the disease.20
Clinical FeaturesThere are 3 clinical phases: the acute phase, from week 0 to week 3; the postacute phase, from week 4 to week 12; and the chronic phase, beyond week 12 after symptom onset.
In 5–18% of the younger patients (<25 years of age) the infection can have an asymptomatic presentation. The incubation period is from 3 to 7 days (range 1–12).
During the acute phase there is high fever (90–95%), polyarthralgia (>80%) and other symptoms like generalized pain, myalgia and exanthema (36–64%).21 The latter is maculopapular, distributed on trunk and extremities, and it develops 2–5 days after the onset of fever. Joint involvement is symmetrical, bilateral, predominantly distal and polyarticular (up to 10 groups of joints). Those most widely affected are interphalangeal joints and the joints of the wrists and ankles. Axial and proximal joints can also be involved and there can even be large joint effusions in knees and shoulders. The condition is disabling and is often accompanied by periarticular edema and tenosynovitis.22 Taking into account all the series studied, it has been observed that approximately 43% of the adult population recovers completely in 3 weeks.
During the outbreak on Reunion Island (2005–2006), there were atypical aggressive neurological manifestations (encephalopathy, flaccid paralysis, Guillain-Barré syndrome), as well as mucocutaneous and respiratory adverse effects, in addition to kidney and liver failure. Although the overall mortality was low, between 0.01% and 0.1%, severe disease was encountered in patients with comorbidities and in those over 75 years of age. Moreover, there was a rate of vertical transmission of 50% when the mother was in the viremic phase (4 days before until 2 days after delivery) and there are cases of spontaneous abortion and neonatal complications (central nervous system involvement, bleeding and myocarditis). Cesarean section did not effectively impede transmission and transmission through breastfeeding has not been demonstrated.23
The symptoms of the postacute phase are similar to those of the acute phase, but are less severe. There are exacerbations of the pain (in the joints affected initially or in others that had not previously been involved), periarticular edema and synovitis.
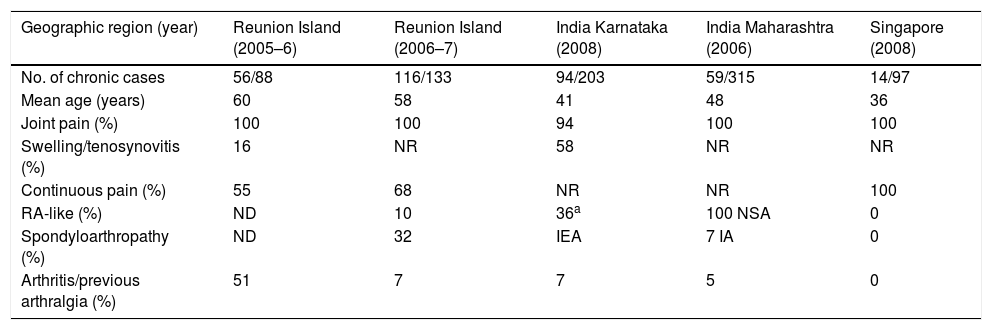
The rheumatic manifestations during the chronic phase after CHIKV infection can have an intermittent course or be persistent. The former are characterized by the fluctuation between periods in which the patients are disease-free and the recurrence of the symptoms, and the latter by continuous, unremitting symptoms.21Table 5 shows the findings in fairly recent studies that evaluated the chronic symptoms of rheumatic disease caused by CHIKV.22,24 The site of the joint involvement during the chronic phase is usually the same as that affected during the acute phase. It is symmetrical and occupies a smaller proportion than the migratory type. Half of the patients (41–79%) report morning stiffness (lasting>30min),14 and some of them mention soft tissue edema, carpal tunnel syndrome, small fiber neuropathy and Raynaud's phenomenon. The periarticular involvement includes tenosynovitis, tendinitis with risk of tendon rupture, enthesitis, bursitis, capsulitis and periostitis. Other associated signs are myalgias, weakness, changes in sleep quality and neuropsychological problems. The joint symptoms can persist for years and are usually accompanied by fatigue, headache or a depressive mood, with an important impact on quality of life.25,26
Chronic Rheumatic Symptoms Induced by Chikungunya Virus (10–36 Months After Acute Phase).
| Geographic region (year) | Reunion Island (2005–6) | Reunion Island (2006–7) | India Karnataka (2008) | India Maharashtra (2006) | Singapore (2008) |
|---|---|---|---|---|---|
| No. of chronic cases | 56/88 | 116/133 | 94/203 | 59/315 | 14/97 |
| Mean age (years) | 60 | 58 | 41 | 48 | 36 |
| Joint pain (%) | 100 | 100 | 94 | 100 | 100 |
| Swelling/tenosynovitis (%) | 16 | NR | 58 | NR | NR |
| Continuous pain (%) | 55 | 68 | NR | NR | 100 |
| RA-like (%) | ND | 10 | 36a | 100 NSA | 0 |
| Spondyloarthropathy (%) | ND | 32 | IEA | 7 IA | 0 |
| Arthritis/previous arthralgia (%) | 51 | 7 | 7 | 5 | 0 |
IA, inflammatory arthritis; IEA, inflammatory and erosive arthritis; ND, not detected or prevalence not evaluated; NR, not reported; NSA, nonspecific arthritis; RA, rheumatoid arthritis.
It is estimated that 5% of the patients who reach the chronic phase will develop true chronic inflammatory rheumatism similar to RA (1/3 with anti-cyclic citrullinated peptide antibodies) or spondyloarthritis (occasionally HLA B27+) or undifferentiated polyarthritis (chronic polyarthritis without meeting criteria for RA or spondylitis).
DiagnosisA “typical” patient is defined as an individual with compatible clinical manifestations (fever>38.5°C and sudden onset arthralgia), with an epidemiological history (as a resident or visitor to regions of local transmission of CHIKV in the preceding 15 days) and with a positive diagnostic microbiological test (polymerase chain reaction [PCR] or serology). A “severe” case is considered to be that in which the patient has a significant dysfunction of at least 1 organ and requires hospitalization.24,27
The diagnostic tests to be performed depend on the duration of the disease. The genomic detection by PCR is the earliest to be done. Specific immunoglobulin (Ig) M antibodies are detected from the 5th day of infection, and those of IgG between the 7th and 10th days and the peak is at 15 days. In persistent disease, IgM antibodies have still been detected up to 6 months after the acute phase. Thus, PCR should be requested between day 0 and the 5th day, serology and PCR between the 5th and 7th day and serology alone starting after the 7th day of infection.28 Other microbiological tests will be requested during the acute phase whenever other agents that can cause similar clinical conditions are suspected (sepsis, dengue, malaria, leptospirosis, etc.).
Other laboratory tests help achieve the diagnosis. During the acute phase, leukopenia, lymphopenia, mild thrombocytopenia and a slight increase in aminotransferase levels can be observed. However, it is not usually necessary to perform ancillary tests unless the patient has atypical or complicated disease. During the postacute phase, it is recommended that tests be performed to rule out other diseases that can cause rheumatic involvement, like autoimmune diseases, gout, chronic hepatitis, thyroid dysfunction, etc. If moderate-to-severe joint effusion is detected during any of the phases of the disease, joint puncture can be performed to confirm its inflammatory nature and exclude other causes (gout, septic arthritis, etc.).
Imaging techniques are not recommended during the acute phase. A radiographic study can be requested in the case of doubts concerning the diagnosis or severe disease with a duration of more than 6 weeks. In the chronic phase, radiographs of the hands, wrists and feet are indicated if the symptoms suggest peripheral disease, or of the pelvis and thoracolumbar spine if the symptoms point to axial disease. Ultrasound can be requested in the case of tendon rupture or soft tissue involvement.27
Treatment and PreventionDuring the acute phase, treatment is mainly analgesic, taking into account the use of drug escalation. Paracetamol is the analgesic of choice, at a maximum of 4g/day.24 The addition of acetylsalicylic acid (ASA) or nonsteroidal anti-inflammatory drugs (NSAID) may be recommended. The former is contraindicated during the first 14 days of infection if dengue has not been excluded as a causative agent (risk of bleeding complications) or the patient is under 12 years of age (risk of Reye syndrome). The use of glucocorticoids is not recommended unless there are complications such as neuritis or encephalopathy. The utilization of opiates like morphine should be reserved only for patients who are seriously ill. Supportive therapy may be necessary in older patients and in children.
The subacute phase is treated with NSAIDs (changing family in the absence of an effect after 10 days of treatment). Systemic glucocorticoids are indicated only in polyarticular disease, particularly when associated with tenosynovitis or active synovitis, or in the case of resistance to or contraindication of the use of NSAIDs. The recommended prednisone dose is 10mg/day for 5 days, to be tapered over 10 days in cases of moderate disease, and 5mg/kg body weight/day, over 5 days, tapered for at least 10 days (maximum 4 weeks) in cases of severe disease, and continuation with NSAIDs to prevent rebound of the symptoms. Joint injections are indicated in patients with capsulitis or synovitis who do not respond to treatment with NSAIDs. General analgesics or drugs for the treatment of neuropathic pain, if present, (pregabalin, gabapentin) and opiates can be taken when the pain is very severe. Ancillary analgesic physiotherapy (pressure therapy, cryotherapy, orthosis, etc.) and the treatment of associated diseases can also be considered.
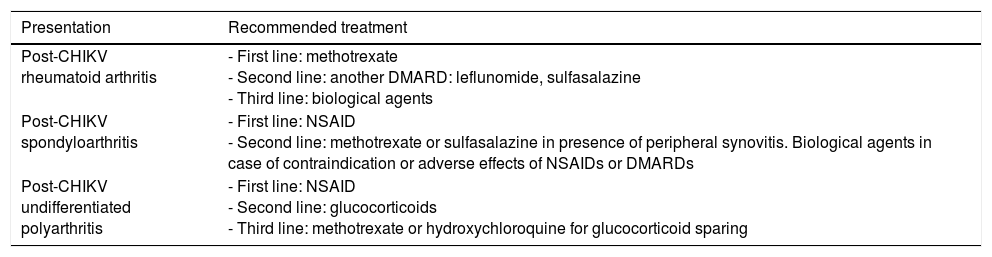
The management of the chronic phase is similar to that of the subacute phase. The patient should be taken to a rheumatologist for evaluation and treatment. Chloroquine has not been found to be effective in chronic musculoskeletal manifestations, although the studies performed had small sample sizes.29 During the outbreak on Reunion Island, many patients were treated with methotrexate and other disease-modifying antirheumatic drugs.30 Ganu et al. reported the outcome in 16 treated patients in whom only 2 (12.5%) had a good response to the combination of hydroxychloroquine and sulfasalazine; 14 required the addition of methotrexate at a dose of 15–20mg weekly in oral or injected pulses, and the response was good in 10 of them (71.4%).31Table 6 shows the treatment of chronic rheumatism after CHIKV infection proposed by the French Society of Rheumatology.27
Treatment of Chronic Post-Chikungunya Rheumatic Disease.
| Presentation | Recommended treatment |
|---|---|
| Post-CHIKV rheumatoid arthritis | - First line: methotrexate - Second line: another DMARD: leflunomide, sulfasalazine - Third line: biological agents |
| Post-CHIKV spondyloarthritis | - First line: NSAID - Second line: methotrexate or sulfasalazine in presence of peripheral synovitis. Biological agents in case of contraindication or adverse effects of NSAIDs or DMARDs |
| Post-CHIKV undifferentiated polyarthritis | - First line: NSAID - Second line: glucocorticoids - Third line: methotrexate or hydroxychloroquine for glucocorticoid sparing |
CHIKV, Chikungunya virus; DMARD, disease-modifying antirheumatic drug; NSAID, nonsteroidal anti-inflammatory drugs.
Source: Simon et al.27
As there is no vaccine or drugs for chemoprophylaxis, prevention is based on the use of barriers during a stay in areas of a high rate of transmission in order to avoid getting bitten by the mosquito vector. These measures include wearing clothing that covers the most exposed parts of the body, the use of insect repellents and sleeping under mosquito nets, preferably impregnated with residual insecticides. During the acute (viremic) phase the altruistic isolation of the patient wherever there are competent vectors could be considered.
Sindbis VirusEpidemiologyInfection by this virus is common in northern Europe, mainly in Finland (Pogosta disease). Outbreaks usually occur every 7 years, mostly between August and September (in relation to vector proliferation), with hundreds or thousands of reported cases. The latest took place in 2002 with 597 cases.32 The main vectors are ornithophilic species of Culex spp. and Culiseta spp., although the virus has also been isolated in Aedes mosquitos and in ticks. As antibodies have been found in birds such as grouse, it has been proposed that they may act as a reservoir.
Clinical FeaturesMost of the infections are asymptomatic. The incubation period is usually 4 days (6–18 days). The typical symptoms include papular exanthema (96%), joint symptoms (96%), fatigue (77%), myalgia (62%) and headache. The presence of fever and upper airway symptoms has been reported in 36% of the patients. Exanthema usually lasts about 6 days and is located on the extremities and trunk. The joints most widely affected are knees, ankles, hands, feet and wrists.33 It usually has little impact on laboratory tests, showing no evidence of changes in complete blood count or coagulation. In patients in whom the symptoms persist (6 months after infection), there may be joint edema, myalgia, tendinitis and serum IgM levels that are still detectable. Recent studies indicate that up to 50% continue to have joint symptoms at 1 year, and that, at 3 years, 25% of them still have joint pain and 4% have arthritis.34
DiagnosisThe diagnosis of the infection is based on the detection of specific IgM and IgG antibodies in the first and second weeks of the disease, respectively. However, they are identified in only 40% of the patients.34 Polymerase chain reaction is usually useless for the diagnosis due to the short time and low level of viremia; it is usually negative, although it could be of value in detecting the virus in mosquitos and potential hosts.32
Treatment and PreventionThe treatment is symptomatic. Prevention is based on the use of barrier measures. No vaccine is available.
O’nyong Nyong VirusEpidemiologyIn the Nilotic languages of Sudan and Uganda, “O’nyong nyong” means “weakening of the joints”. This virus was first isolated in 1959 in Uganda. Since then, there have been 3 large epidemics. The first, which occurred in 1959–1962, affected more than 2 million people, and the last 2 were in 1996 and 2002, respectively.35 This virus has genetic origins similar to those of CHIKV and many infections by O’nyong nyong virus have been attributed to the latter. However, in contrast to other alphaviruses, it is transmitted by Anopheles mosquitos (Anopheles gambiae and Anopheles funestus), which are also malaria vectors. No animal reservoir has been identified and it is thought that humans are the only hosts.
Clinical FeaturesIn half of the cases, the course of the infection is asymptomatic. The incubation period is usually 8 days. The disease is characterized by sudden onset fever, headache, symmetric polyarthralgia or arthritis, and generalized papular or maculopapular exanthema (60–70%). There can also be pharyngeal pain, eye pain, chest pain, general malaise, lymphadenopathy–often on the back of the neck–and red eye syndrome in 50% of the patients.36 Mild leukopenia may be detected during the acute phase. The duration of arthralgia is usually 1 week, but there have been cases of its lasting up to 90 days. The joints most widely affected are knees (90%), ankles (83%), shoulders (75%), wrists (75%) and fingers (63%). The pain is disabling, impeding mobilization for up to 28 days in some cases. No deaths secondary to this infection have been reported.
DiagnosisThe virus can be amplified by PCR from the third day of the disease. Specific IgM antibodies are detected, with a peak that occurs 21 days after the onset of the disease and persists up to 1 month. In addition, IgG antibody levels are elevated starting in the third week after the onset of symptoms. In terms of serology, it displays cross reactivity to CHIKV.
Treatment and PreventionTreatment is only symptomatic. The drug of choice is paracetamol, alone or associated with a NSAID. The use of repellents and mosquito nets are the usual measures to avoid being bitten by the mosquito. There is no available vaccine.
Ross River VirusEpidemiologyThe virus was isolated for the first time in 1963 in mosquitos collected near the Ross River in Townsville in the north of Queensland, Australia. This virus can cause disease both in humans and animals (horses). It is transmitted among marsupial mammals, which are the reservoir. The main vectors are Aedes vigilas, Aedes camptorhynchus and Culex annulirostris, which are confined to Australia and to the Asia-Pacific region.37 The incidence of RRV infection is 5000–8000 cases each year. In June 2015, a total of 7552 cases was declared (the highest number since 1996). The peak of transmission is between the months of February and April.38 In Australia, the disease is endemic to the north (Queensland) and is seasonal or epidemic in the south (New South Wales). It also affects other regions like Papua New Guinea, East Timor and the Solomon Islands.
Clinical FeaturesThe disease mainly affects adults between 20 and 60 years of age, and those having a previous rheumatic disease are more susceptible. The infection is rare in children and it is presumed that this is due to a difference in the immune response when compared with adults. Ross River virus is capable of affecting the signaling pathway of osteoblasts causing an increase in bone resorption.39
The incubation period is about 9 days (3–21 days). The clinical manifestations begin suddenly with disabling arthralgia, accompanied by maculopapular exanthema (66%) on extremities and trunk, which lasts from 5 to 10 days, constitutional symptoms (50%) and fever (33%). Laboratory tests show an increase in the erythrocyte sedimentation rate with no relevant changes in the complete blood count. Those infected may feel unable to work for the first 2–3 months. The acute phase of the disease resolves slowly over the successive months, but remissions and exacerbations of the symptoms (arthralgia, enthesitis, joint effusions, myalgia, fatigue) are frequent and last more than a year.40–42
DiagnosisThe diagnosis can be based on the detection of specific antibodies. However, this has the disadvantage of displaying cross-reactivity with other similar viruses, such as dengue, which circulates in the same region. The IgM response occurs at 7–10 days of infection and the peak is at 2–3 weeks. Using PCR techniques, it is possible to amplify the virus during the first 7 days of the disease in blood or in the synovial fluid.37
Treatment and PreventionTreatment is based mainly on support measures and analgesics (paracetamol and NSAIDs). The effectiveness of the treatment was evaluated in a prospective study involving 255 patients, and it was found that 36.4% achieved relief from the symptoms with a NSAID and only 16.4% improved with paracetamol or aspirin. Physical therapy (swimming, hydrotherapy or massages) was beneficial in 10% of the patients, whereas in 24% it was the only treatment that relieved the symptoms.43 The use of repellents and mosquito nets are the usual measures to prevent mosquito bites. No vaccine is available.
Barmah Forest VirusEpidemiologyThis virus is endemic in Australia (where it was isolated for the first time in 1974, in Victoria) with an incidence of 13.2 per 100,000 population. It is seasonal, with a peak of transmission from February to April.44,45 The main vectors are A. vigilax, Aedes procax and A. camptorhychus and, in urban environments, Aedes notoscriptus and C. annulirostris. Given that RRV and Barmah Forest virus (BFV) share the same vector and the same geographic distribution, it is not rare to encounter people with mixed infections involving these 2 viruses. Although neutralizing antibodies have been found in many vertebrates (marsupials, horses, dogs), experimental infection in these animals produces a very low level of viremia, meaning that the vector is unable to acquire the virus.46,47
Clinical FeaturesMost of the infections are subclinical or unapparent. Infection can occur at any age but the population most widely affected is that comprised of people from 45 to 64 years old.
The disease has an incubation period of 5–21 days. The onset is sudden, with fever, disabling arthralgia and maculopapular exanthema.47 The latest outbreak of BFV occurred in February 2002. It involved 40 patients: 95% had arthralgia, 72.5% exanthema, 62.5% headache, 52.5% myalgia, 52.5% fever and 42.5% chills.48 Polyarthralgia is very common and the joints most widely affected are the knees, wrists, ankles and those of the hands. Cases have been reported of joint effusion and monocytes have been identified. There is usually a slight elevation of inflammatory markers like C-reactive protein and erythrocyte sedimentation rate. There are usually no changes in the complete blood count. Arthralgia persists for more than 6 months in up to 10% of the patients.49
DiagnosisThe diagnosis is based on the detection of specific IgM antibodies or IgG seroconversion. Up to 19% of the patients had cross-reactions with other viruses. Techniques like PCR enable the detection of genetic material from the virus during the first days of the disease.44
Treatment and PreventionOnly symptomatic treatments like NSAIDs and paracetamol are utilized. The use of repellents and mosquito nets are the usual measures to prevent mosquito bites. No vaccine is available.
Mayaro VirusEpidemiologyIsolated for the first time in Trinidad in 1954, this is a typical disease of the rural areas of South America, especially in the northern region and the Amazon basin.50 It is considered that the reservoir of the virus are birds, lizards and certain mammals like monkeys. Transmitted by mosquitos of the Culicidae family, mainly by the Haemagogus janthinomys, that feed during the daytime. The vector inhabits tropical rainforests, near the niches of the monkeys, and the female places the eggs in hollows in trees, in cut bamboo and artificial recipients like tires and cans. Experimental studies have demonstrated that A. aegypti, Aedes scapularis and A. albopictus can be infected by the virus and, effectively, be transmitted and, thus, could potentially cause urban outbreaks.51,52
Clinical FeaturesThe incubation period is not clearly defined in the literature, but some authors indicate that it is 8 days. The clinical onset is sudden, with the development of fever (100%), arthralgia (50–89%), myalgia (75%), headache (64–100%), joint edema (58%), exanthema, which appears on the fifth day (32–49%) and retroocular pain (44–63%). Other less common manifestations include pruritus, dizziness, weakness, lymphadenopathy, jaundice and vomiting.50,53 There may be abnormal findings in the complete blood count, such as leukopenia and thrombocytopenia, but they are not important. Arthralgia affecting wrists, ankles, elbows, knees and fingers may persist as long as 12 months and interfere with activities of daily living and work, and there can even be joint effusion and stiffness.50,54
DiagnosisSpecific serological tests can be performed to detect IgM and IgG. Polymerase chain reaction is useful during the early days of the disease. Serological tests may result in cross-reactions with other alphaviruses.
Treatment and PreventionOnly symptomatic treatment with NSAIDs and paracetamol is employed. The use of repellents and mosquito nets are the usual measures to prevent mosquito bites. No vaccine is available.
Conclusions- •
The most important alphaviruses associated with polyarthritis are the Sindbis, Ross River, Mayaro, O’nyong nyong and Barmah Forest viruses, but the indisputable prototype is Chikungunya.
- •
In contrast to other viruses, they affect the bones, skeletal muscle, myotendinous insertions and joint capsules.
- •
The incubation period is short, less than 14 days. During the acute phase (weeks 0–3), patients develop fever, polyarthralgia, myalgia and exanthema. Half of them recover completely in 3 weeks. Some patients enter in a chronic phase (>12th week), with osteoarticular symptoms that can persist for years. Five percent will develop true chronic inflammatory rheumatism similar to RA or spondyloarthritis, or undifferentiated polyarthritis.
- •
The diagnosis is based on PCR during the first week and later on serological tests.
- •
The treatment of choice is paracetamol, which can be accompanied by ASA or a NSAID. Glucocorticoids are indicated in the presence of polyarticular disease, tenosynovitis, active synovitis or in the case of resistance to or contraindication of the NSAIDs. In the chronic phase, hydroxychloroquine, sulfasalazine, methotrexate or other disease-modifying antirheumatic drugs can be used according to the guidelines.
- •
The patient should be referred to a rheumatologist for evaluation and treatment.
The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Mejía C-R, López-Vélez R. Alfavirus tropicales artritogénicos. Reumatol Clin. 2018;14:97–105.