There has recently been an increase in the incidence of patients with systemic lupus erythematosus (SLE) mainly due to earlier diagnosis, and increased survival. Tuberculosis in our country is one of the most prevalent infectious diseases, and one of the underlying causes would be HIV infection and increased immigration from areas with high tuberculosis prevalence; this phenomenon is truly important in patients with autoimmune diseases, as clinical presentation, severity and prognosis of tuberculosis are often different to that of immunocompetent patients. Studies of tuberculosis in patients with SLE are scarce and inconclusive, with many doubts existing about the performance or non-tuberculous prophylaxis in this population and the absence of a protocol due to lack of conclusive studies. New techniques for diagnosis of tuberculosis (IGRAs) may be useful in this population due to higher sensitivity than Mantoux, helping avoid false negatives.
En la actualidad se ha detectado un aumento en la incidencia de pacientes con lupus eritematoso sistémico (LES) debido fundamentalmente a un diagnóstico más precoz, e incremento en la supervivencia de estos. La tuberculosis en nuestro país es una de las enfermedades infecciosas más prevalentes; como causa subyacente, entre otras, estarían la infección por VIH y el aumento de inmigrantes procedentes de áreas con alta prevalencia tuberculosa; este fenómeno es verdaderamente importante en los pacientes con enfermedades autoinmunes, ya que la presentación clínica, intensidad y pronóstico de la tuberculosis suelen ser diferentes a los de los pacientes inmunocompetentes. Los estudios sobre tuberculosis en paciente con LES son escasos y poco concluyentes, habiendo hoy en día muchas dudas sobre la realización o no de profilaxis tuberculosa en esta población y sin haberse establecido aún un protocolo de actuación por falta de estudios concluyentes al respecto. Las nuevas técnicas de diagnóstico de infección tuberculosa (IGRA) podrían ser útiles en esta población debido a su mayor sensibilidad respecto al Mantoux, evitando también los falsos negativos del mismo.
Tuberculosis is the most common infectious disease in the world, estimated at about 8 million new cases per year, with 12 million existing cases, involving a total of 20 million cases annually.1 Consequently, the general population is more exposed to the TB bacilli, something particularly disturbing in immunocompromised persons, including patients with systemic lupus erythematosus (SLE),2 and this phenomenon is exacerbated in developing countries that have detected a high incidence of extrapulmonary tuberculosis with high mortality3 figures.
In Spain the actual incidence of tuberculosis in the general population is not well known due to, among other causes, underreporting and underdiagnosis, especially among the elderly population.4 In recent years there has been a decline in the number of reported cases, reaching 5795 in 2007, although it is estimated that approximately 30%–40% of cases are not reported, so the numbers could be significantly higher.5 According to data from the National Epidemiological Surveillance Network, the total number of cases reported in 2009 was 7652, equivalent to a crude incidence rate of 16.96 cases per 100000 persons. Communities with higher overall rates were Ceuta, Galicia, La Rioja and Melilla, although the number of reported cases include Catalonia, Andalusia, and Madrid.6
Currently, the main causes of death in patients with lupus are cardiovascular7 disorders and infections, the latter predominating in several series.8 The administration of steroids and cyclophosphamide are 2 of the main risk factors in the development of infection.9,10 Differential diagnosis between infection and lupus flares is sometimes difficult, but essential because delays in treatment of both processes are associated with increased mortality.11 Moreover, TB simulates some of the findings of autoimmune diseases such as joint pain/arthritis, rash or presence of autoantibodies, which poses a problem for the diagnosis of this disease.12
In patients with SLE there are immunologic and genetic alterations that predispose them to the development of infections.13 Among those described are a deficit in complement, functional asplenia, impaired phagocytic system or functional deficits in the activity of T cells, with reduced cytotoxic T cells and alterations in the function of suppressor T cells, determining increased susceptibility to infections by mycobacteria.14 Added to this, the use of immunosuppressive agents such as azathioprine, cyclophosphamide or mycophenolate produces decreased T and B lymphocytes counts, and the widespread use of corticosteroids blocks T cell proliferation, cytotoxic T cells, microbicidal activity and antigen-specific immune response, producing a significant impairment of cellular immunity, and increased risk of infection by intracellular pathogens such as Mycobacterium tuberculosis, viruses of the herpes family and Pneumocystis jirovecii among other pathogens such as Listeria spp. and Nocardia spp.15
In countries where TB is endemic there have been numerous studies in patients with lupus. However, in Spain these are scarce and inconclusive.3,4,16
In a retrospective study comprising 390 patients with SLE from the Philippines,12 13.8% had active tuberculosis, and of these, 74% had only pulmonary involvement; it also found that patients with disseminated infection had a higher lupus activity index and more aggressive disease, regardless of the corticosteroid dose they were receiving at the time of diagnosis. In another retrospective17 study that included 283 SLE patients and 284 with rheumatoid arthritis from Korea, it was found that the most common form of involvement was pulmonary tuberculosis and lupus, mainly in patients with higher doses of corticosteroids. Alongside this, a greater percentage of extrapulmonary tuberculosis was described in lupus patients than in the general population, up to 45% of cases by one of the published series,18 and the main risk factor was higher daily or cumulative doses of corticosteroids during the year before diagnosis, more often for arthritis and renal involvement. These findings were similar to those described by Tam et al.,19 who conducted a case-control study in Hong Kong consisting of 526 patients with SLE an who provided analysis of the different risk factors involved in the development of active tuberculosis. In this study, a univariate analysis, patients with SLE and cerebral involvement had higher rates of tuberculosis, nephritis, vasculitis, cumulative dose of corticosteroids and intravenous methylprednisolone pulses relative to the control group. In the multivariate analysis, the presence of nephritis and the cumulative dose of corticosteroids were independent risk factors for developing the disease. They also found an increase of extrapulmonary tuberculosis, which together with the military for, was the most common presentation in patients (67%). The other risk factor independently associated with the occurrence of tuberculosis in patients with SLE was pleuritis.20
As for Spanish studies on tuberculosis in patients with lupus, there have been three types so far. The first was a retrospective study which consisted of patients with lupus treated in the Systemic Autoimmune Diseases Unit of the Hospital de Cruces in the Basque Country between 1994 and 2003.3 In this, the incidence of active tuberculosis in patients with SLE (187 cases per 100000) was compared with the population of the area (20–45 cases per 100000), which turned out to be much lower than that of patients with lupus, and no predictor for the development of tuberculosis was identified among their patients. The second, also retrospective, was held at the Rheumatology Service, Hospital Clinico San Carlos in Madrid, and was composed of patients diagnosed with autoimmune disease and tuberculosis treated at the Unit.4 They found an incidence rate of tuberculosis in patients with autoimmune diseases of 153 cases per 100000 patient-years, which increased to 645 cases/100000 patient years when analyzing patients with SLE and vasculitis; in that year the incidence of tuberculosis in the Autonomous Community of Madrid was 26 cases per 100000 population per year, concluding that extrapulmonary tuberculosis was more common in patients with autoimmune disorders in the general population. Tuberculosis patients were older and had no other risk factors for developing the disease. The third study was made up of a cohort of 789 (January 1989–January 2009) SLE patients treated in the Autoimmune Diseases Unit of the Hospital Universitario Virgen del Rocío de Sevilla, comparing patients with and without tuberculosis.16 They found 13 cases, with 61% having extrapulmonary forms. Overall mortality attributed to this infection was 30.8% and the frequency of tuberculosis was higher than that in the general population. However, although patients treated with higher doses of corticosteroids had more severe forms of the disease, no statistically significant differences were seen with other immunosuppressive therapies or greater frequency of renal involvement.
The problem of the different epidemiological studies that have been conducted so far is that most are retrospective, introduce selection bias, and do not take into account socio-economic conditions.
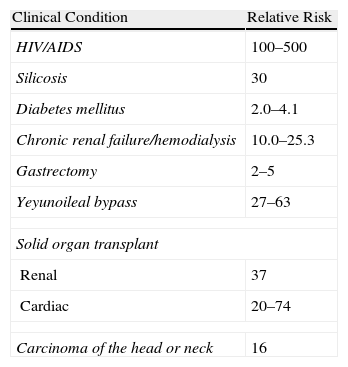
With respect to the diagnosis and treatment of latent tuberculosis, general recommendations, according to the American Thoracic Society,21 consider the need for treatment with isoniazid for 9 months when the skin reaction (TST) is ≥10mm in patients with diseases that increase the risk of developing the infection, and ≥5mm in all those receiving more than 15mg of prednisone daily or its equivalent. It also recommended annual TST for all persons whose social condition merited it or presented an increased risk of active tuberculosis during certain time periods or in the foreseeable future.22 This risk is well established for certain groups of patients, but not known exactly in lupus patients (Table 1).
Relative Risk With Respect to the Population for the Development of Active Tuberculosis.
| Clinical Condition | Relative Risk |
| HIV/AIDS | 100–500 |
| Silicosis | 30 |
| Diabetes mellitus | 2.0–4.1 |
| Chronic renal failure/hemodialysis | 10.0–25.3 |
| Gastrectomy | 2–5 |
| Yeyunoileal bypass | 27–63 |
| Solid organ transplant | |
| Renal | 37 |
| Cardiac | 20–74 |
| Carcinoma of the head or neck | 16 |
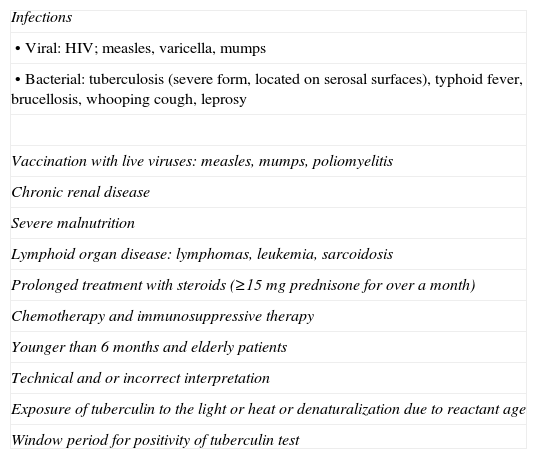
In Spain, the Society of Clinical Microbiology and Infectious Diseases, along with the Society of Pneumology and Thoracic Surgery (SEPAR), recently published a consensus document on recommendations for diagnosis, treatment and prevention of tuberculosis.23 This document contains the instructions for the TST (Table 2), in which patients with prolonged immunosuppressive therapy are included, and with causes associated with false negatives, such as prolonged corticosteroid therapy (≥15mg of prednisone for more than one month), and any immunosuppressive chemotherapy (Table 3).
Indications of Tuberculin Testing.
| Contacts of tuberculosis patients |
| Persons with X rays suggestive of inactive tuberculosis |
| Persons with clinical and/or radiological signs of tuberculosis |
| Persons who, if infected and have a greater risk for the development of tuberculosis |
| • HIV infection |
| • IV drug users |
| • Homeless persons |
| • Foreigners from countries with high prevalence |
| Immunosuppressive diseases: leukopenia, lymphoma, neoplasia, and others |
| Prolonged immunosuppressive therapy, anti-TNF-α and patients who are candidates for transplant |
| Patients with social and epidemiological risk of developing active tuberculosis |
| • Day care center workers |
| • Teaching staff |
| • Health staff |
| • Prison staff |
| Epidemiologic studies and antituberculosis control programs |
Causes of a False Negative Result on the Tuberculin Test.
| Infections |
| • Viral: HIV; measles, varicella, mumps |
| • Bacterial: tuberculosis (severe form, located on serosal surfaces), typhoid fever, brucellosis, whooping cough, leprosy |
| Vaccination with live viruses: measles, mumps, poliomyelitis |
| Chronic renal disease |
| Severe malnutrition |
| Lymphoid organ disease: lymphomas, leukemia, sarcoidosis |
| Prolonged treatment with steroids (≥15mg prednisone for over a month) |
| Chemotherapy and immunosuppressive therapy |
| Younger than 6 months and elderly patients |
| Technical and or incorrect interpretation |
| Exposure of tuberculin to the light or heat or denaturalization due to reactant age |
| Window period for positivity of tuberculin test |
Today, new tuberculosis diagnostic techniques are being introduced into clinical practice guidelines from various societies, but their role in the various risk groups is yet to be determined. They are based on the detection of gamma interferon in the blood (interferon gamma release assay [IGRA]), which is released in response to in vitro stimulation of primed T-cells with specific antigens of M. tuberculosis. There are currently two commercialized tests: QuantiFERON®-TB Gold In-Tube, using ELISA, and T-SPOT®. TB, based on the ELISPOT24 technique. The concordance rate between the two is high, but it seems that T-SPOT®. TB is more sensitive than QuantiFERON®-TB Gold In-Tube.25 At present, SEPAR recommends a Mantoux test for diagnosis of tuberculosis infection in immunocompromised patients, and if negative, the performance of an IGRA (to avoid false negatives due to immunosuppression). For SLE patients, some studies have been published using QuantiFERON®-TB Gold in areas of high prevalence of tuberculosis.26,27 Their authors have been struck by the high percentage of indeterminate results of this technique with respect to the general population, due to lymphocytopenia suffered by many of these patients and the degree of disease activity, so they recommend interpreting the results with caution. In Spain there are still no data.
Despite the recommendations, and that the incidence of tuberculosis in lupus appears to be higher than in the general population,16–19 the relative risk of infectious reactivation has not been formally established in these patients,11 and therefore the use of isoniazid for treating latent tuberculosis in lupus patients is not formalized. Regarding this issue, two studies have been published in high TB prevalence areas, India28 and Hong Kong.29 The first found a reduction in the prevalence of active tuberculosis in lupus patients following administration of isoniazid, 11%–2%. In the second there was no greater protection, which we believe was due to the wrong design and methodology, since isoniazid was administered intermittently depending on the steroid dose received by the patient.
In conclusion, we can say that tuberculosis is currently one of the most common infectious problems worldwide, and it is especially necessary to detect the latent phase in patients with autoimmune diseases because of the risk of reactivation. Regarding patients with SLE and tuberculosis in Spain, there is little data and it is all retrospective, so we need prospective studies on incidence and more viable and diagnostic clinical trials to evaluate the risk/benefit of treatment of latent infection.6,17,25
Ethical ConsiderationsProtection of Persons and AnimalsNo experiments were performed on humans or animals.
Data ConfidentialityPatient data do not appear in this article.
Right to Privacy and Informed ConsentAuthors obtained informed consent from patients and/or subjects referred to in this paper. These are in the hands of the corresponding author.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Arenas Miras MM, et al. La infección tuberculosa en pacientes con lupus eritematoso sistémico: situación en España. Reumatol Clin. 2013;9:369–372.