The association between systemic lupus erythematosus (SLE) and thrombotic thrombocytopenic purpura (TTP) has been infrequently reported. Usually, patients with TTP have more SLE activity and frequent renal involvement. Here we present a case of TTP associated to low-activity SLE. The absence of renal and major organ involvement increased the difficulty in making the initial diagnosis. ADAMTS13 activity in plasma in this patient was very low, as seen in other similar cases. The evolution of the patient was poor, needing plasma exchanges and immunosuppressive therapy, including the use of rituximab.
La asociación entre lupus eritematoso sistémico (LES) y púrpura trombótica trombocitopénica (PTT) es una situación descrita pero poco frecuente y suele ocurrir en casos con actividad lúpica y deterioro renal. Presentamos un caso de PTT con disminución de los niveles de ADAMST13 que ocurrió en una paciente afectada de LES pero sin actividad y sin afectación renal. Fue un caso que presentó dificultades diagnósticas iniciales y que, debido a su refractariedad, precisó recambios plasmáticos y tratamientos inmunosupresores, incluyendo rituximab.
Thrombotic thrombocytopenic purpura (TTP) is characterized by the presence of fever, microangiopathic hemolytic anemia, thrombocytopenia and neurological and kidney disorders.1,2 Although most cases are idiopathic, familial cases and cases are associated with neoplasia, infection, drugs, pregnancy and collagenosis may be seen.3 Idiopathic TTP is due to the presence of inhibitory antibodies against the ADAMTS132,4,5 metalloprotease.
TTP is a serious condition with a high mortality rate if untreated. Treatment with plasma exchange has greatly improved the prognosis and mortality has decreased from 85%–100% to 10%–30%.2,5,6 However, there are refractory cases and relapse occurs in 40% of patients. In these situations various immunosuppressive agents, including rituximab (RTX) have been employed.7,8
TTP is a rare complication in the context of systemic lupus erythematosus (SLE). The cases described in medical literature generally occur in patients with severe lupus activity and renal involvement.9 We present a case of a patient with SLE, with good disease control of lupus activity, who abruptly developed an episode of TTP of a refractory and aggressive course and who required plasma exchange, as well as additional immunosuppressive therapy, including RTX.
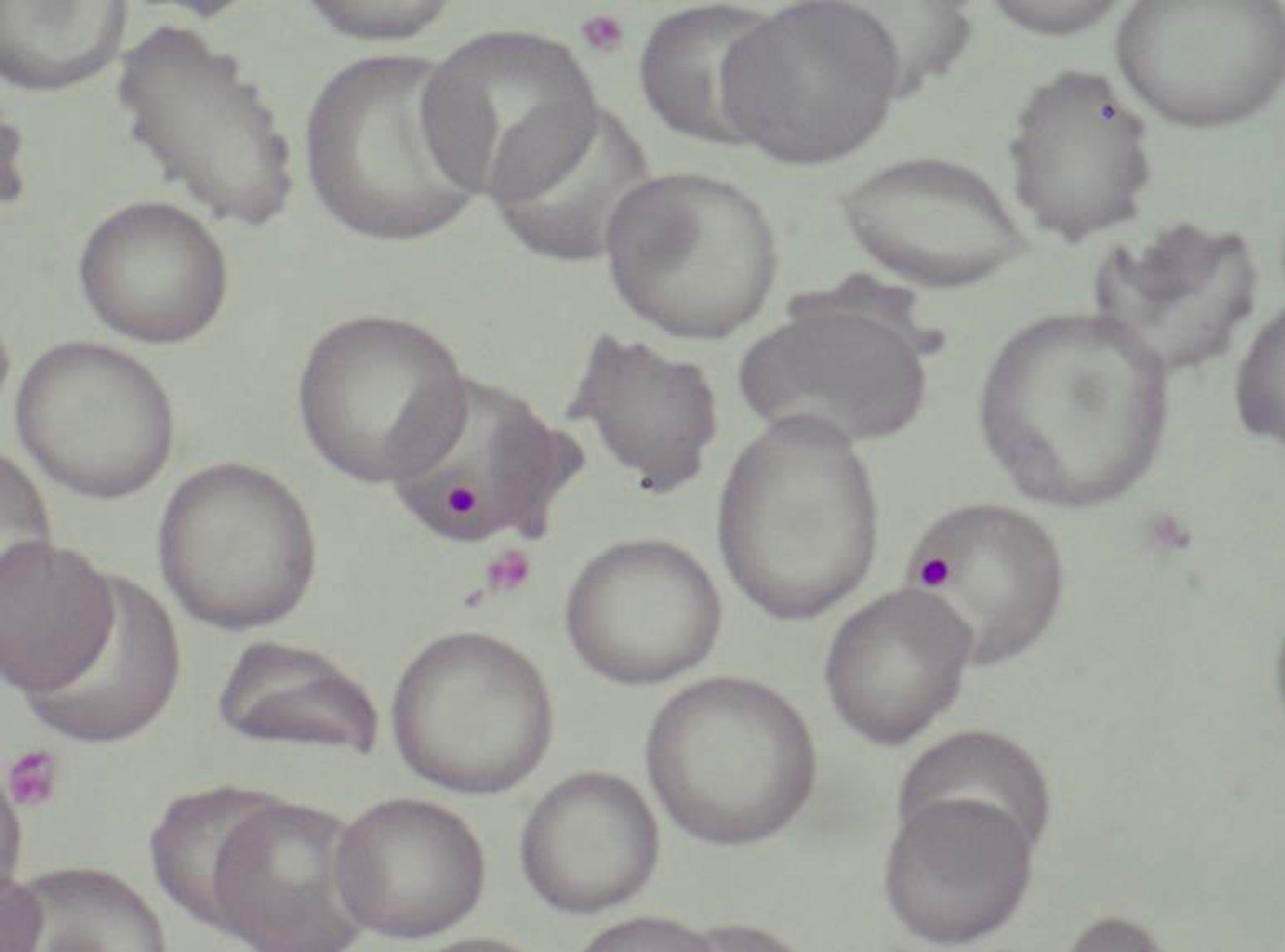
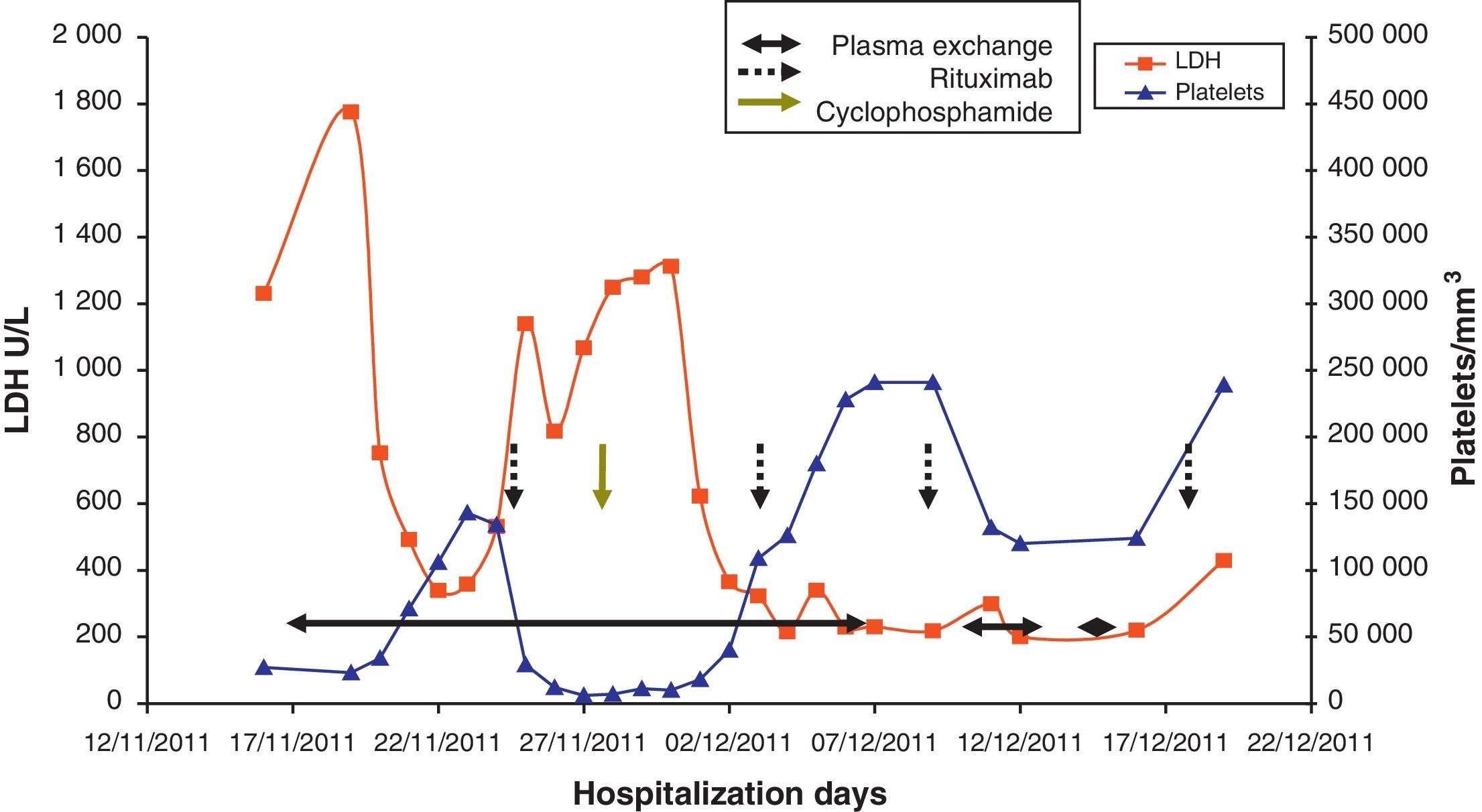
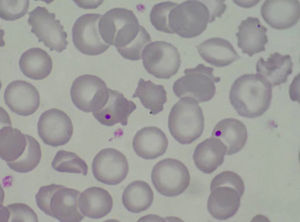
Case PresentationThe patient is a 47 year old woman who came to the emergency department in December 2011 due to nonspecific epigastralgia which had started several days earlier. The patient had been diagnosed with SLE in 1998 presenting palindromic arthritis, oral ulcers, anti-DNA positive serology and positive antinuclear antibodies, as well as a left pleural effusion and pericarditis in 2001. In the last four years the patient has shown no clinical signs of lupus activity, receiving treatment with prednisone 10mg/d, hydroxychloroquine 200mg/12h omeprazole. In the ER the clinical examination revealed a good general condition, without neurological, cardiac or pulmonary alterations nor fever or blood pressure abnormalities, with some bruising in the abdomen and arms. The blood tests on admission showed: hemoglobin 10.5g/dl, platelets 27,000mm–3, LDH 1231U/l, gamma glutamyl transpeptidase 70U/l, alanine aminotransferase 54U/l, total bilirubin 1.40mg/dl and CRP32mg/l, with normal urea and creatinine. Complementary examinations, electrocardiogram, chest and abdominal X-rays and abdominal ultrasound were normal. Because of hematologic and biochemical abnormalities suggestive of hemolysis and a thrombocytopenia probably of immune origin, the patient was admitted for evaluation, treated with an increase in the usual dose of prednisone0.5mg/kg. During the first days of hospitalization there was a worsening of thrombocytopenia down to 17,000plateletsmm–3, and 4 days later the patient presented a sudden neurological language deficit and loss of strength in her right hand, which fully recovered after a few hours. A cerebro-vascular MRI revealed multiple acute lacunar infarcts in the right cerebellar and left frontal hemisphere. In subsequent laboratories, proteinogram, thyroid function, renal parameters and urine biochemistry were normal. Determinations of lupus anticoagulant and direct Coombs test were negative, antinuclear antibody 1/160 (normal<1/80) and haptoglobin<10. The Hematology Department confirmed a Coombs negative anemia with hemolytic characteristics (increased LDH, indirect bilirubin and reticulocytes with decreased haptoglobin). We reviewed the peripheral blood morphology, detecting schistocytes with increased basophilic staining of the erythrocytes and Howell-Jolly bodies (Fig. 1), confirming microangiopathic hemolytic anemia. The thrombocytopenia and neurological alterations led to a final clinical diagnosis of TTP. We immediately began high dose steroids (1.5mg methylprednisolone per kg body weight per day) and plasma exchange (40ml/kg every day), suspending hydroxychloroquine treatment. After three plasma exchange sessions, platelet counts and LDH levels were normalized, but two days later these parameters deteriorated again, reaching a platelet count <10000mm–3. Given the resistance to treatment, we decided to add a second line therapy with RTX 375mg/m2 weekly for 4 weeks. After the first dose of RTX, we suspended the plasma exchange for 48h to increase its effectiveness, but after 24h without plasmapheresis the patient had a further decline in hemoglobin (Hb: 5.6g/dl) and LDH increased in schistocytes in the peripheral blood and new neurological symptoms consisting of confusion, motor aphasia and weakness. We decided to reinstall treatment with daily plasma exchange and administered pulse IV cyclophosphamide (750mg/m2). Neurological symptoms ceded and the patient recovered quickly after 2 days with platelet levels of LDH improving to normal. After 4 days with complete response, we began to space the plasma exchange and suspended it after a total of 21 sessions, while the pattern of steroids was reduced to 10mg/d of prednisone. At 4 months after discharge she remains in complete hematologic remission, requiring no treatment other than low-dose prednisone. MTS13 ADA levels obtained at baseline were 0% and 2 months after the end of treatment remain the same. Fig. 2 shows the development of the disease process and treatments used.
TTP is characterized by microangiopathic hemolytic anemia, thrombocytopenia, fluctuating neurological and renal disorders. The occlusion of arterioles and capillaries by microthrombi composed essentially of platelets is typical of this condition and is due to increased platelet aggregation associated with the presence of large multimers of von Willebrand factor (Fv W), presumably due to the decreased activity of the ADAMTS13 enzyme, responsible for cleaving these multimers.2,4,5 It has been suggested that the pathogenesis of TTP in SLE may be related to inhibiting the action of metalloproteinase by autoantibodies to ADAMTS1310 but no unanimous agreement exists in this respect.
Although the association of SLE and PTT is described in the literature, there is an infrequent association and constitutes a difficult diagnosis. Recently, a series of 283 patients with TTP collected over 20 years has been published,6 including determinations of levels of ADAMTS13.It was considered low when there was <10% of normal ADAMTS13 activity. In the study group, 107 cases were idiopathic (37.8%), 21 cases (7.4%) secondary to lupus and 16 cases (5.6%) secondary to other immune processes. ADAMTS13 level were low in almost half of idiopathic cases (48%), but only in 2 patients with SLE (10%) and in one with other immunological diseases (7%) was it found decreased. When the authors reviewed the clinical associations of idiopathic cases they proved that those with low levels of ADAMTS13 were younger (41 years vs 57 years) and had lower platelet count at diagnosis (17000mm–3 vs 47000mm–3), and more chance of renal involvement, needing more plasma exchange sessions (19 vs 9) and having more chance of recurrence (40% vs 7%). In another study, Letchumanan et al.,9 studied 10 cases of idiopathic TTP and 8 cases of TTP associated with lupus. Typically, the SLE associated TTP cases occurred in a phase of disease activity and had more renal involvement. These data indicate that the case presented here has a profile similar to idiopathic TTP with ADAMTS13 suppression.6 The age profile, the more pronounced thrombocytopenia, the absence of renal involvement, the greater number of plasma exchanges required and refractoriness all match. However, the onset of the process had an atypical behavior as lupus activity showed no renal involvement at the time of diagnosis.9 In other respects, our case showed two major problems caused by the association between SLE and TTP. The first is the difficulty in diagnosis, because the clinical presentation may be mistaken for signs of SLE itself: hemolytic anemia, thrombocytopenia and abdominal pain. The blood smear and the detection of schistocytes is essential to categorize microangiopathic hemolytic anemia. The absence of a positive Coombs’ test, along with the other symptoms, thrombocytopenia and neurological involvement support the diagnosis of TTP and require urgent plasma exchanges. Low levels of ADAMTS13 further corroborate the diagnosis of TTP. Letchumannan et al., comparing idiopathic TTP cases and those associated with lupus, reported that it takes almost 2 weeks longer to diagnose cases associated with SLE cases (19.5 vs 7.9 days).9 The second problem is the worse response to treatment. In the same study cited, researchers found that the response time was slower in SLE secondary cases than in idiopathic TTP (31.3 days vs 16.8 days), making it necessary to use higher doses of cytotoxic immunosuppressive and biological agents, and that mortality was higher in the group with SLE.9 In our patient first-line therapy failed, making it necessary to add an initial plasma exchange scheme and more aggressive corticosteroid treatment with immunosuppressive agents which have shown good results in these cases, such as RTX5,11,12 and cyclophosphamide.13
In summary, we review the case of a patient with a rare association of TTP and SLE associated with a decrease in ADAMTS13 activity. The case is noteworthy because the patient did not have lupus activity and because of the difficulty in the early diagnosis and the aggressive immunosuppressive treatment required.
Ethical ResponsibilitiesProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and /or subjects mentioned in the article. The author for correspondence is in possession of this document.
DisclosuresThe authors have no disclosures to make.
Please cite this article as: Garcia Boyero R, et al. Lupus eritematoso sistémico y púrpura trombótica trombocitopénica: un caso refractario sin actividad lúpica asociada. Reumatol Clin. 2013;9:373–375.