To describe the 2-year incidence of new extra-articular manifestations (uveitis, psoriasis, inflammatory bowel disease) in a cohort of patients with spondyloarthritis included in the AQUILES study.
PatientsOver a period of 2 years, 513 patients with spondyloarthritis (62.5% males, mean age 48 years) diagnosed with ankylosing spondylitis (AS) (55.6%), psoriatic arthritis (25.3%), undifferentiated spondyloarthritis (16.2%), enteropathic arthritis (2.5%), and other diseases (0.4%) were followed. New diagnoses were based on reports of the corresponding specialists (ophthalmologists, dermatologists, gastroenterologists).
ResultsDuring the 2-year follow-up, 22 new diagnoses of the extra-articular manifestations were established, with a cumulative incidence of 4.3% (95% confidence interval 2.4–6.1) and an incidence rate of 17 cases per 10,000 patient-year. Uveitis was the most frequent diagnosis (cumulative incidence 3.1%), predominantly in patients with AS. In the multivariate analysis, the diagnosis of AS was the only predictive variable associated to the development of new extra-articular disease.
ConclusionsIn patients with spondyloarthritis, the 2-year global incidence of uveitis, psoriasis and inflammatory bowel disease (IMID) was 4.3%, particularly due to new diagnoses of uveitis in patients with AS.
Describir la incidencia a los 2 años de nuevas manifestaciones extraarticulares (uveítis, psoriasis, enfermedad inflamatoria intestinal) en la cohorte de pacientes con espondiloartritis del estudio AQUILES.
PacientesDurante 2 años se siguieron 513 pacientes con espondiloartritis (62,5% varones, edad media 48 años) diagnosticados de espondilitis anquilosante (EA) (55,6%), artritis psoriásica (25,3%), espondiloartritis indiferenciada (16,2%), artritis enteropática (2,5%) y otros diagnósticos (0,4%). Los nuevos diagnósticos se basaron en los informes de los respectivos especialistas (oftalmólogos, dermatólogos y gastroenterólogos).
ResultadosSe establecieron 22 nuevos diagnósticos de las manifestaciones extraarticulares estudiadas (incidencia acumulada: 4,3% [intervalo de confianza del 95% 2,4-6,1]; tasa de incidencia: 17 casos por 10.000 pacientes-año). La uveítis fue el diagnóstico más frecuente (incidencia acumulada del 3,1%), predominantemente en pacientes con EA. En el análisis multivariable, el diagnóstico de EA fue el único predictor de aparición de nueva manifestación extraarticular.
ConclusionesEn pacientes con espondiloartritis, la incidencia de uveítis, psoriasis y enfermedad inflamatoria intestinal a los 2 años fue globalmente del 4,3%, principalmente por nuevos diagnósticos de uveítis en pacientes con EA.
Spondyloarthritis (SpAs) constitute a heterogeneous group of chronic inflammatory rheumatic diseases with a prevalence of 1.5%–2% of the general population.1,2 However, estimates vary depending on the prevalence of the HLA B27 allele, having established in a French population that its positivity increases 39 times the risk of suffering from SpA compared to HLA B27-negative subjects.3 In a recent study on the prevalence of ankylosing spondylitis (AS) at a worldwide level, figures by 10.00 inhabitants from 23.8 in Europe, 16.7 in Asia, 31.9 in North America, 10.2 in Latin America and 7.4 in Africa have been described.4 SpAs typically present several manifestations in the axial and peripheral skeleton (spondylitis, arthritis, enthesitis and dactylitis,) but they are usually accompanied by extra-articular manifestations,5,6 including uveitis, inflammatory bowel disease (IBD), mucocutaneous injuries and cardiovascular and renal impairments,7 which determines important connotations in the patients’ diagnosis, treatment and follow-up.8 Psoriasis, uveitis and IBD are diseases with their own entity that may appear and evolve independently from the SpA, and are a part of a wider group of conditions that have also been named “immune-mediated inflammatory diseases”.9–11 In addition to phenotypic manifestations, they share with the SpAs a strong familial aggregation, common pathogenetic mechanisms and some genetic determinants, such as HLA B27, NOD2 and ATG6L1 genes in the case of the SpA and IBD and polymorphisms in the IL-23R gene in psoriasis, AS and Crohn's disease.12–14 Although the frequency of these 3 diseases in patients diagnosed with SpA is more elevated than in the general population,10,15 there is not much information on their prevalence and incidence in the SpA.16 The purpose of this study was to describe the incidence of new diagnoses of the 3 aforementioned extra-articular manifestations (uveitis, psoriasis and IBD) in the cohort of patients with SpA included in the AQUILES study at the 2-year follow-up.
Patients and MethodsThe AQUILES study was a prospective and observational analysis of 3 independent cohorts of patients (SpA, psoriasis and IBD) defined by the diagnosis of the main disease at the time of their inclusion in the study. The investigation was carried out in 15 hospitals in Spain, with the participation of the Rheumatology, Dermatology and Digestive System departments, in conditions of usual clinical practice. The screening period extended from March 2008 to December 2010. The protocol was approved by the participating hospitals’ ethics committees and the study was performed complying with the standards of good clinical practice. Patients were included in the study in accordance with the main diagnosis made by rheumatologists, dermatologists or gastroenterologists, and the follow-up was performed by the same specialist who included them in the cohort. Baseline data referring to the 3 cohorts of patients have been previously published.17–19
The purpose of this study was to determine the incidence of psoriasis, uveitis and IBD in the 2-year follow-up in the cohort of patients with SpA. This cohort included adult patients, aged 18 years or older, for whom a rheumatologist has established the diagnosis of SpA, including AS, psoriatic arthritis (PA), undifferentiated SpA, arthritis associated to IBD or others. At the time of recruiting the subjects (2008–2010), the current term axial SpA had not been coined yet, which is why it is not gathered in the classification of our patients. The diagnosis of SpA could have been made before (that is, already known in the past) or de novo in patients visited for the first time in the Rheumatology office of the participating hospitals. Patients that, at the investigator's discretion, presented any issue preventing their follow-up for a period of 2 years were excluded from the cohort. In regards to the 3 extra-articular manifestations (uveitis, psoriasis, IBD), the diagnosis could appear in the medical records, due to being present at baseline or because the patient had suffered a previous outbreak (for example, uveitis) and, as such, diagnosis had been entered in the medical record. The follow-up of the cohort with SpA was performed by their rheumatologists and only in the case there was a clinical suspicion of extra-articular affectation, the patient was referred to other specialists (the protocol did not include a scheduled review in all patients). New diagnoses were based on the reports from the corresponding specialists, ophthalmologists for uveitis, dermatologists for psoriasis and gastroenterologists for IBD.
Qualitative variables are described with their absolute frequency and their percentage. Continuous variables are presented with the mean and standard deviation or with the median and interquartile range (25 and 75 percentiles) when they did not adjust to a normal distribution. The differences in the accumulated incidence of extra-articular manifestations in the different entities of SpA were analysed with the Pearson's Chi-Square Test (χ2). A logistic regression model was used to determine the clinical and demographic variables independently associated to the incidence of new extra-articular manifestations, presenting the adjusted odds ratio (OR) and the confidence interval (CI) of 95%. The statistical significance was established with a P-value below .05. The results were analysed with the Statistical Package for the Social Sciences® version 15.0 for Windows®.
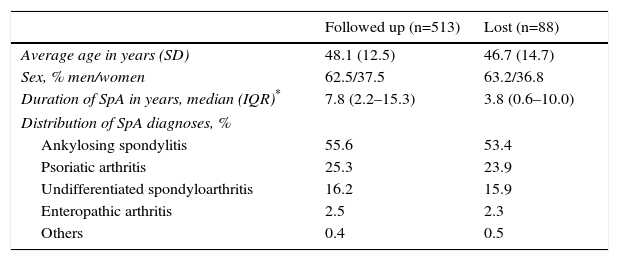
ResultsA total of 601 patients were included in the SpA cohort of the AQUILES study, 513 (85.3%) of them completed the 2-year follow-up, who are those analysed in this study. Most losses during follow-up were due to the change of specialist. There were no significant differences in baseline characteristics between the 513 patients included in the follow-up and the 88 patients excluded, except for a longer duration of the disease in the followed-up patients (Table 1). The average age of the study population was 48.1 years (standard deviation 12.5); 62.5% were men and 37.5% were women.
Characteristics of the Patients Followed up During 2 Years and Those Lost.
| Followed up (n=513) | Lost (n=88) | |
|---|---|---|
| Average age in years (SD) | 48.1 (12.5) | 46.7 (14.7) |
| Sex, % men/women | 62.5/37.5 | 63.2/36.8 |
| Duration of SpA in years, median (IQR)* | 7.8 (2.2–15.3) | 3.8 (0.6–10.0) |
| Distribution of SpA diagnoses, % | ||
| Ankylosing spondylitis | 55.6 | 53.4 |
| Psoriatic arthritis | 25.3 | 23.9 |
| Undifferentiated spondyloarthritis | 16.2 | 15.9 |
| Enteropathic arthritis | 2.5 | 2.3 |
| Others | 0.4 | 0.5 |
SD, standard deviation; SpA, spondyloarthritis; IQR, interquartile range.
25.9% were smokers and 30.8% revealed alcohol consumption (moderate in 90.2% of the cases). 12.1% of the cases were diseases diagnosed at the time of entering the study, and 22.3% of the patients had a history of SpA in the direct family. 13.5% of the patients presented extra-articular manifestations different to the studied ones (conjunctivitis [4.5%], cystitis [2.3%], ungula hyperkeratosis [5.3%], balanitis [1.2%], and <1% presented pulmonary fibrosis, aortic insufficiency or renal amyloidosis). SpA diagnoses at the beginning of follow-up were as follows: AS in 285 cases (55.6%), PAs in 130 (25.3%), undifferentiated SpA in 83 (16.2%), enteropathic arthritis in 13 (2.5%) and others in 2 (0.4%). The mean duration of SpA was 7.8 years (interquartile range 2.2–15.3 years). The percentages of patients with different treatments for the SpA at inclusion in the study were: non-steroidal anti-inflammatory drugs (63.5%), glucocorticoids (8.8%), disease-modifying antirheumatic drugs (21.6%) and biologic drugs (34.7%). In patients with AS, the percentages in treatment with non-steroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs and biologic drugs were 74.3, 2.0 and 10.5% respectively, while in PAs they were 39.4, 25.4 and 13.4%, respectively.
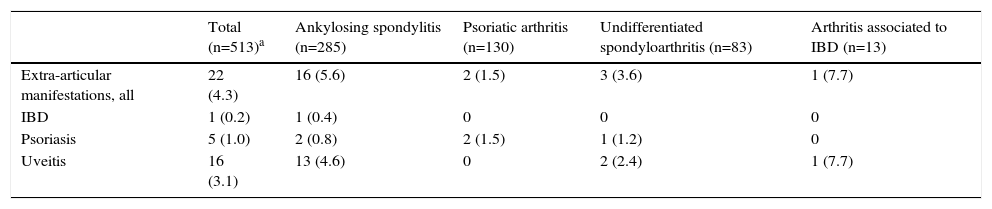
By the 2-year follow-up, 22 new diagnoses of the 3 extra-articular studied manifestations had been established, with an accumulated incidence of 4.3% (CI of 95% 2.4–6.1) and an incidence rate of 17 cases by 10 000 patients-year. Each extra-articular manifestation's accumulated incidence and incidence rates are shown in Table 2. The prevailing diagnosis was uveitis, with a total of 16 cases (3.1% accumulated incidence, CI of 95% 1.5–4.7). Moreover, 16 (72.7%) of the 22 new diagnoses of extra-articular manifestation were observed in patients with AS (Table 3). On the other hand, most patients with PAs already presented psoriasis at baseline and only 2 were diagnosed with psoriasis during follow-up, without any other accompanying incidents of extra-articular manifestations (Table 3).
Accumulated Incidence at 2-Year Follow-up of New Extra-articular Manifestations in the Cohort of Patients With Spondyloarthritis From the AQUILES Study.
| Extra-articular manifestations | Number of incident cases | Incidence at 2 years, % (CI of 95%) | Incidence rate by 10 000 patients-year (CI of 95%) |
|---|---|---|---|
| All | 22 | 4.3 (2.4–6.1) | 17.3 (11.5–26.2) |
| IBD (ulcerative colitis) | 1 | 0.2 (0–1.1) | 0.8 (0.1–4.5) |
| Psoriasis | 5 | 1.0 (0.2–2.3) | 4.0 (1.7–9.2) |
| Uveitis | 16 | 3.1 (1.5–4.7) | 12.6 (7.8–20.5) |
IBD, inflammatory bowel disease; CI, confidence interval.
Accumulated Incidence at 2 Years of Follow-up of New Extra-articular Manifestations in the Cohort of Patients With Spondyloarthritis From the AQUILES Study Based on the Base Spondyloarthritis.a
| Total (n=513)a | Ankylosing spondylitis (n=285) | Psoriatic arthritis (n=130) | Undifferentiated spondyloarthritis (n=83) | Arthritis associated to IBD (n=13) | |
|---|---|---|---|---|---|
| Extra-articular manifestations, all | 22 (4.3) | 16 (5.6) | 2 (1.5) | 3 (3.6) | 1 (7.7) |
| IBD | 1 (0.2) | 1 (0.4) | 0 | 0 | 0 |
| Psoriasis | 5 (1.0) | 2 (0.8) | 2 (1.5) | 1 (1.2) | 0 |
| Uveitis | 16 (3.1) | 13 (4.6) | 0 | 2 (2.4) | 1 (7.7) |
IBD, inflammatory bowel disease.
Data expressed as n (%).
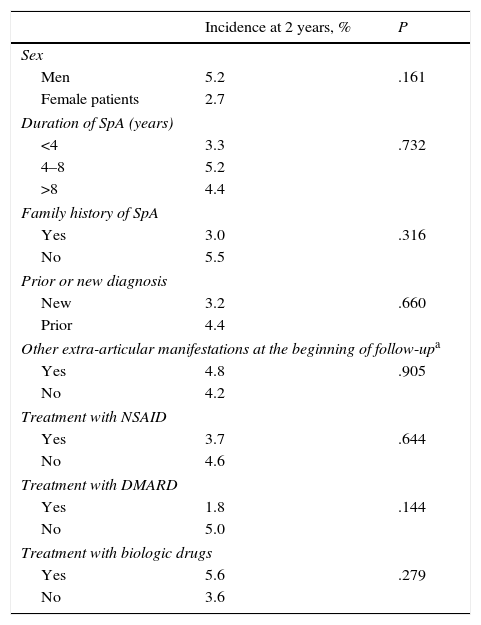
No significant differences were found in the incidence of new extra-articular manifestations at the 2-year follow-up in the comparisons between men and women, presence or absence of familial disease or other extra-articular manifestations from the beginning of follow-up, as well as differences based on disease duration or on the treatments that patients were receiving (Table 4). In the multivariable analysis, the only independent predictive factor of development of other extra-articular manifestations was the diagnosis of AS compared to the PAs (OR 3.5; CI of 95% 1.0–15.3; P=.049).
Incidence After 2 Years of New Extra-articular Manifestations in Subgroups of Patients.
| Incidence at 2 years, % | P | |
|---|---|---|
| Sex | ||
| Men | 5.2 | .161 |
| Female patients | 2.7 | |
| Duration of SpA (years) | ||
| <4 | 3.3 | .732 |
| 4–8 | 5.2 | |
| >8 | 4.4 | |
| Family history of SpA | ||
| Yes | 3.0 | .316 |
| No | 5.5 | |
| Prior or new diagnosis | ||
| New | 3.2 | .660 |
| Prior | 4.4 | |
| Other extra-articular manifestations at the beginning of follow-upa | ||
| Yes | 4.8 | .905 |
| No | 4.2 | |
| Treatment with NSAID | ||
| Yes | 3.7 | .644 |
| No | 4.6 | |
| Treatment with DMARD | ||
| Yes | 1.8 | .144 |
| No | 5.0 | |
| Treatment with biologic drugs | ||
| Yes | 5.6 | .279 |
| No | 3.6 | |
NSAIDs, non-steroidal anti-inflammatory drugs; SpA, spondyloarthritis; DMARDs, disease-modifying antirheumatic drugs.
The results obtained after 2 years of follow-up in a cohort of 513 patients with SpA show the possibility of appearance of other immunity-mediated inflammatory diseases during the course of the condition and confirm that uveitis is the extra-articular manifestation with the highest incidence (16 out of the 22 new diagnoses [72.7%]), mostly in patients with AS. Uveitis is one of the most frequent extra-articular manifestations in patients with SpA.20 Additionally, approximately 40% of the patients with prior uveitis present undiagnosed SpA at the time of the outbreak.21 In this sense, close cooperation between ophthalmologists and rheumatologists is of paramount importance in the long-term diagnosis and prognosis of uveitis. However, there is no consensus regarding the need for systematic clinical screening or whether it is convenient to perform a routine ophthalmoscopic examination in patients with SpA. Our study did not include a specific detection strategy, so it is possible that some mild subclinical or asymptomatic outbreaks of uveitis may have gone unnoticed, and the accumulated incidence of uveitis after 2 years may have been underrated. Most cases in our series (81.2%, 13/16) were diagnosed in patients with AS, probably because they are a wide majority in the cohort, but we should not forget that it is also the most frequent extra-articular manifestation in PAs after psoriasis,22 so patients with this disease should be subject to special care.
The accumulated incidence of psoriasis was only 1.0%, since practically every patient with PAs had already been diagnosed with psoriasis at the beginning of the study. However, we should mention that arthritis precedes psoriasis in 10%–15% of the patients,23 usually as an undifferentiated SpA, and skin affectation in these patients may take months or years to appear. In this sense, the patient should be instructed to see the dermatologist if there is presence of skin alterations and, especially, ungual lesions.
The appearance of new diagnoses of IBD was very rare, since only one patient with AS developed ulcerative colitis. We must bear in mind that the IBD incidence rate in the Spanish population is very variable depending on the geographic region, and that the methodology used in epidemiological studies is still very heterogeneous.24 However, as in the rest of the Occidental world, the Spanish population studies published since 2000 gather an increase in incident cases in some regions, until stabilisation with estimates that vary between 4.2 and 12.5/100 000 inhabitants-year for ulcerative colitis and 0.8–10.5/100 000 for Crohn's disease.25 Therefore, the incidence in our hospital cohort would be within the range described in the general population, but it is very likely that the sample size is not capable of detecting differences and we cannot rule out subclinical profiles that do not manifest themselves because of the treatments with proved efficiency in both diseases.
However, we did not find differences in the incidence of extra-articular manifestations based on the treatment received by the patients. Although some studies show that certain manifestations such as uveitis may decrease with the use of some disease-modifying antirheumatic drugs or biologic drugs,26,27 the short duration of the follow-up or the fact that precisely the most-symptomatic patients are those more treated could mask the potential effect of these drugs.
As limitations to this study, it is important to mention that patients were followed up in the Rheumatology practice, and they were only referred to other specialists in cases of concomitant disease, without establishing of a follow-up protocol by other specialists or an active search protocol of extra-articular manifestations through additional diagnostic tests for all the patients, but only when they presented indications of disease. Hence, some diseases such as uveitis or even certain very mild cases of IBD or psoriasis could have certain infradiagnosis. Additionally, although the patients were seeing in conditions of clinical practice, all of them belonged to a hospital environment, so they could not be representative of the general population of patients with SpA. On the other hand, it would have been interesting to analyse a more homogeneous cohort, for example, only those patients with AS or undifferentiated forms, since most patients with PAs already have psoriasis and those who have enteropathic arthritis already have IBD. Finally, in this study, we did not gather the HLA-B27 antigen status, so another limitation is that we cannot affirm whether the incident cases of uveitis occurred in patients with positive HLA-B27.
In conclusion, patients with SpA, especially those with AS, may develop immunity-mediated inflammatory diseases, especially uveitis, during the chronic course of their base disease. That determines a clinical profile with expressiveness in different systems and organs, which forces a comprehensive approach to treatment and diagnosis of the patient with SpA.
Ethical ResponsibilitiesProtection of people and animalsAuthors state that no experiments were performed on human beings or animals as part of this investigation.
Confidentiality of dataAuthors state that this article does not contain patient data.
Right to privacy and informed consentAuthors state that this article does not contain patient data.
FundingThe AQUILES study was funded by Merck Sharp & Dohme de España, S.A.
Conflict of InterestsRosario García-Vicuña: consultant in hospital project for MSD; expenses associated to scientific congresses. Francisco Vanaclocha: none. Pedro Zarco: advisor and medical education programs for MSD, AbbVie and Pfizer. Carlos M. González: grant MSD, advisor or medical education programs for MSD, AbbVie, Pfizer, Roche and UCB. Ignacio Marín-Jiménez: presentations or educational projects for MSD, AbbVie, Falk Pharma y Shire, consultancy for MSD, Ferring, Falk Pharma, AbbVie, FAES and Shire. Luis Cea-Calvo: employee at MSD, Spain.
We hereby thank the investigators for their cooperation in the inclusion and follow-up of study patients. The statistical analysis was performed by Cristina Fernández Pérez (Alalás, S. A.) and the document writing received the cooperation of Dr. Marta Pulido and Content Ed Net Communications, S. L., Madrid.
Please cite this article as: García-Vicuña R, Zarco P, González CM, Vanaclocha F, Marín-Jiménez I, Cea-Calvo L. Incidencia a los 2 años de psoriasis, uveítis y enfermedad inflamatoria intestinal en la cohorte de pacientes con espondiloartritis del estudio AQUILES. Reumatol Clin. 2016;12:22–26.