The pharmacologic management of rheumatoid arthritis has progressed substantially over the past years. It is therefore desirable that existing information be periodically updated. There are several published international guidelines for the treatment of rheumatoid arthritis that hardly adapt to the Mexican health system because of its limited healthcare resources. Hence, it is imperative to unify the existing recommendations and to incorporate them to a set of clinical, updated recommendations; the Mexican College of Rheumatology developed these recommendations in order to offer an integral management approach of rheumatoid arthritis according to the resources of the Mexican health system.
ObjectiveTo review, update and improve the available evidence within clinical practice guidelines on the pharmacological management of rheumatoid arthritis and produce a set of recommendations adapted to the Mexican health system, according to evidence available through December 2012.
MethodsThe working group was composed of 30 trained and experienced rheumatologists with a high quality of clinical knowledge and judgment. Recommendations were based on the highest quality evidence from the previously established treatment guidelines, meta-analysis and controlled clinical trials for the adult population with rheumatoid arthritis.
ResultsDuring the conformation of this document, each working group settled the existing evidence from the different topics according to their experience. Finally, all the evidence and decisions were unified into a single document, treatment algorithm and drug standardization tables.
ConclusionsThis update of the Mexican Guidelines for the Pharmacologic Treatment of Rheumatoid Arthritis provides the highest quality information available at the time the working group undertook this review and contextualizes its use for the complex Mexican health system.
El manejo de la artritis reumatoide ha tenido avances muy importantes en los últimos años. Las guías de práctica clínica requieren una actualización constante. Recientemente se han publicado diversas guías internacionales para el manejo farmacológico de la artritis reumatoide que difícilmente se adaptan a la realidad mexicana, en especial por la heterogénea disponibilidad de los medicamentos en las diversas instituciones del sector salud. Por ello, debido a la importancia de unificar el criterio de manejo con los tratamientos disponibles, el Colegio Mexicano de Reumatología decidió revisar las guías existentes e incorporar nueva evidencia actualizada y adaptada a la realidad del sistema de salud mexicano.
ObjetivoRevisar, actualizar y adaptar la guía del manejo farmacológico de la artritis reumatoide y emitir recomendaciones adaptadas al sistema de salud de México, de acuerdo con manejos disponibles hasta diciembre de 2012.
MétodoParticiparon en la elaboración de la guía 30 reumatólogos certificados con experiencia y juicio clínico. Las recomendaciones se basaron en niveles de evidencia de las guías de tratamiento previamente establecidas, ensayos clínicos controlados y guías estandarizadas para población adulta con artritis reumatoide.
ResultadosDurante la conformación del documento, cada grupo de trabajo estableció la evidencia existente sobre los diferentes temas a tratar según su campo de mayor experiencia clínica, siendo enriquecida por la opinión de los demás expertos. Al final, toda la evidencia y las decisiones tomadas se unificaron en un manuscrito, se desarrolló un algoritmo de tratamiento y se resumieron en tablas estandarizadas por medicamento.
ConclusionesLa actualización de la Guía Mexicana para el Tratamiento Farmacológico de la Artritis Reumatoide integra la mejor información disponible para la toma de decisiones y contextualiza su empleo al complejo y heterogéneo sistema de salud mexicano.
The development of this update of these Mexican guidelinelines for the Pharmacological Treatment of rheumatoid arthritis has the objective of maintaining the validity of the recommendations established by the previously published guidelinelines and to serve as a framework for clinical decision-making based on the best available current evidence, with the added objective of standardizing practices for treating rheumatoid arthritis (RA) in adults and improving the quality and safety of medical care for this condition.
JustificationRA is a public health problem worldwide due to its high prevalence, its serious functional consequences and high economic and social impact.
In Mexico, it is estimated that RA has a prevalence of 1.6%1 and mainly affects the age group with higher labor and production capacity, which is reflected in the high rates of work disability and disability pension that have a high impact on the economy, not to mention the decline in the quality of life of patients.2
It is estimated that the direct medical cost in dollars of RA in Mexico is $2334, and patient pocket spending is $610. It has been found that 15% of household income is spent on RA related expenses, which is considered as catastrophic for the family economy.3 RA's annual direct medical cost is estimated at $5944.4
An appropriate and timely treatment increases the chance of limiting the progression of joint damage and, consequently, improving the functionality and quality of life for patients and reducing the economic impact it generates. Therefore, in Mexico there is a need to adapt and disseminate recommendations regarding the treatment of RA based on the complex reality of the Mexican health system.
In 2009, in order to show the most current scientific evidence, to assist in making an assessment and timely diagnosis as well as guiding and standardizing both pharmacological and non-pharmacological effective treatment based on the latest and best scientific evidence treatment, guidelinelines for Clinical Practice, Diagnosis and Treatment of Adult Rheumatoid Arthritis in Mexico were developed.5 However, because new clinical research findings provide updated evidence, a need to update them arose, and the Mexican College of Rheumatology decided to review the existing guidelinelines and incorporate new scientific evidence based on the Canadian guidelinelines for Treatment of RA.
UsersThe updated Mexican guidelinelines for the Pharmacologic Treatment of Rheumatoid Arthritis is directed at rheumatologists. It may be helpful to other specialists who eventually engage, in an interdisciplinary manner, in the management of patients with RA.
MethodologyEstablishment of the Working Group- •
August 2012: meeting of experts of the Mexican College of Rheumatology to define objectives, to evaluate the content of the recommendations and determine the update process through a universal and consensual approach to this objective.
- •
September 1, 2012. Planning meeting where relevant issues were agreed upon and a directed selection of coordinators based on membership in the National System of Researchers with expertise in the subject, in the process of publication, with geographic, gender and potential conflict of interests balance was carried out. Each of them was asked to incorporate 3 rheumatologists to their teams, taking into account, among other criteria, residency training centers, the different health systems, private practice, academic profile and the different regions of the country.
- •
September 8, 2012: It was decided to adopt and adapt the Canadian guidelinelines for treatment of RA, as a summary of the various published guidelinelines on the management of RA.6,7 This guidelineline was enriched by the addition of new structured clinical questions that the group of coordinators developed by consensus at that meeting.
The participants identified the issues to assess and the questions to respond based on the most common treatment problems facing health care professional, focused mainly on the pharmacological treatment of RA in adults.
Development of RecommendationsSearch ProtocolThe search process included: clinical practice guidelinelines (CPG), randomized clinical trials and meta-analyzes, published in the period between January 2010 and September 2012, in English or Spanish, in the adult population (aged 18 years), regardless of gender.
The search was conducted in PubMed, the Cochrane Library and specialty websites, and performed with the descriptor “Arthritis, Rheumatoid” in relation to MeSH subheadlines “Therapy” and “Drug therapy”.
Specialized websites consulted were: National guidelinelines Clearinghouse, Tripdatabase, NHS Evidence, Alberta Medical Association guidelinelines, Australian Government, National Health and Medical Research Council, American College of Physicians, Scottish Intercollegiate guidelinelines Network, Institute for Clinical Systems Improvement Ministry of Health of Chile, Singapore MOH guidelinelines, CMA Infobase, NZGG, NICE, Health guideline and Canadian Rheumatology Association.
As a result of this systematic process of information, 372 search results were found. The algorithm selection protocol algorithm is shown below. 372 articles identified through the search protocol 261 articles were excluded by title and abstract according to whether they complied with the objectives of the guidelineline update Preliminary selection of 111 articles for review 86 (7 GCP) articles selected for review according to current evidence:
However, it is a document based on a non-systematic review that includes practical recommendations on the treatment of RA in Mexico. However, they are recommendations: the degree of agreement for the 30 rheumatologists who conducted was not established, nor subjected to external evaluation by rheumatologists on each of the 37 clinical situations that are answered.
Development of Evidence and RecommendationsThe presentation of the evidence and recommendations for updating this guidelineline corresponds to the information obtained from international GPC, where the criteria used to select were those used by the Appraisal of guidelinelines for Research and Evaluation II (ADREE II),8 according to their methodological quality for their adoption and adaptation, taking as its starting point the following guidelinelines:
| No. | Title | ADREE II score (%) |
| 1. | The management of rheumatoid arthritis in adults. NICE | 83.3 |
| 2. | Management of early rheumatoid arthritis. SIGN | 81.9 |
| 3. | Canadian Rheumatology Association Recommendations for the Pharmacological Management of Rheumatoid Arthritis with Traditional and Biologic Disease-modifying Antirheumatic Drugs | 80.2 |
| 4. | Canadian Rheumatology Association Recommendations for the Pharmacological Management of Rheumatoid Arthritis with Traditional and Biologic Disease-modifying Antirheumatic Drugs: Part II Safety | 80.2 |
| 5. | EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying anti rheumatic drugs | 75.2 |
| 6. | EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases | 73.8 |
| 7. | Update of the clinical practice guidelinelines for the management of rheumatoid arthritis in Spain | 72.2 |
The evidence and recommendations from the GPC used as reference documents maintained the degree in accordance to the original scale used. In the case of not having GPC as a reference document, evidence and recommendations were developed through critical analysis of the scientific literature (systematic reviews, meta-analyzes, randomized clinical trials and observational studies), for which the National Institute for Health and Care Excellence (NICE)9 scale was used, in order to deliver the level of evidence (LE) and the degree of recommendation (DR).
The teams reviewed the following issues: (1) analgesics, NSAIDs and glucocorticoids, (2) role of traditional disease modifying antirheumatic drugs (DMARDs), (3) anti-tumor necrosis factor (TNF) therapy, (4) new biologics (abatacept, rituximab, tocilizumab) (5) tofacitinib, and (6) safety and special cases.
Two members of the safety team (AGG, MVDR) did a scrutiny of publications sent as a result of the literature identified in the search, in order to select those that contemplate safety issues and provide new information, thus identifying 21 articles. In 11 of these, data that corresponded to the questions were found, and were included in the recommendations, independent of whether previously established in the Canadian Rheumatology Association (CRA) guidelinelines.6,7
In addition, 2 other publications were included. One corresponded to the publication of the Mexican registry (because it contains national data that were published in the period in which the search was conducted): Biologic therapy: survival and safety in rheumatic diseases. Results from the Biobadamex National Registry 1.0.10 The other was on a systematic review of biologic therapy and pregnancy, also published during the search.11
The evidence was classified numerically and recommendations alphabetically, both in decreasing order according to the type of study design and methodological quality.
Development of Recommendations- i.
Review of the evidence following the methodology and the previously proposed search protocol.
- ii.
Face to face meeting for presentation, discussion and voting on each of the recommendations contained in this guideline.
- iii.
Assessment of guidelinelines with the ADREE II method.
- iv.
Performance of the pharmacotherapy algorithm.
- v.
Standardized summary of information per drug. Line of treatment, paraclinical studies required before and during use, combinations or monotherapy, time to define dose adjustment and drug withdrawal, minimum and maximum doses, contraindications and use in special cases. It also included information on cyclophosphamide, azathioprine, and d-penicillamine (www.paracelsus.mx/algorithm/anexo.docx).
- vi.
Evaluation and approval of the final document.
The guideline is aimed at second and third level of care personnel with the objective of standardizing the actions concerning the pharmacological treatment of RA.
The application of these guidelinelines will improve the effectiveness, safety and quality of health care of this population. The different phases of dissemination and implementation of clinical recommendations should be properly planned and supervised by the respective regulations and regulatory bodies, as well as the respective government agencies.
ExonerationThis paper presents a series of recommendations based on the best scientific evidence available at the time of its development and is intended as a useful tool to expedite decision making for treatment, according to the best available scientific evidence available, always considering the needs and individual patient preferences, and the availability of local resource consumption of the clinic and/or institution. It is important to consider that new clinical research results provide current evidence, so in general, the performance of an update of the same is recommended every 2 years, especially now that the biotech biosimilar/biocomparable drugs are incorporated into our health system.
Abbreviations
Anti-CCP: anti-citrullinated peptide antibodies
BC: blood chemistry
CBC: complete blood count
CLQ: chloroquine
DMARDs: disease modifying antirheumatic drug
DR: degree of recommendation
HCQ: hydroxychloroquine
HIV: human immunodeficiency virus
IDRA: interferon gamma release assays
IFX: infliximab
JAK: Janus kinase
LE: level of evidence
LEF: leflunomide
LFT: liver function tests
MTX: methotrexate
NICE: National Institute for Health and Care Excellence
NSAIDs: nonsteroidal anti-inflammatory
PPD: purified protein derivative
RA: rheumatoid arthritis
RFT: renal function tests
RTX rituximab
SSZ: sulphasalazine
TB: tuberculosis
TCZ: tocilizumab
TNF: tumor necrosis factor
RA is a chronic autoimmune disease that affects 1.6% of Mexican adults1 and is more prevalent in women, with a ratio of 6:1.12 If not diagnosed early or treated promptly, it can lead to disability, a decline in the quality of life and life expectancy, and can lead to high cost-expenditure for the patient, family and society.13 Improvement on the knowledge of its pathogenesis has been significant, as has ongoing drug development in recent 15 years. This requires medical societies to periodically review and update, in a critical manner, the place that these therapeutic options should have in medical decisions.
The guidelinelines provide the physician with current medical evidence to propose an optimal and rational treatment. The Mexican College of Rheumatology, aware of the need to update their guidelinelines, presents new information in the form of recommendations that include the latest drugs approved for this indication and contextualizes their employment, taking into account the reality of the Mexican health system. Possible implementation barriers that may be useful to identify limitations and try to optimize the treatment of this disease are discussed.
The working group emphasizes the importance of early diagnosis and prompt treatment,14 and identifies serious limitations that prevent these two concepts from becoming a reality in Mexico. Likewise, it recognizes the importance of treatment by a rheumatologist with an objective of attaining remission or at least a state of low clinical activity.15 We recognize the importance of comprehensive management of RA, but this guideline focuses on the pharmacological treatment, which could be one of the guidelines’ limitations.
Questions Addressed| Treatment strategies |
| 1. What are the general principles of management of RA?2. What are the goals of treatment? |
| Treatment with analgesics, anti-inflammatory drugs and glucocorticoids |
| 3. What is the role of analgesics, NSAIDs, glucocorticoids and neuromodulators in the treatment of RA?4. Can an NSAID or glucocorticoid be administered with MTX? |
| Treatment with conventional DMARDs |
| 5. When should therapy with DMARDs be initiated?6. What DMARDs should be considered as first choice?7. What laboratory studies are required before starting treatment with MTX and during follow-up?8. Is there evidence in the literature that the parenteral application of MTX increases efficacy compared with its oral administration?9. Is it safe to use MTX in patients with interstitial lung disease?10. When should traditional DMARDs be combined?11. Is there evidence in the literature to pass from DMARD combination therapy to monotherapy once the therapeutic response has been achieved?12. What is the role of cyclophosphamide in the treatment of RA? |
| Treatment with anti-TNF biologic DMARDs |
| 13. What is the role of anti-TNF treatment in RA?14. In patients with active RA who are using anti-TNF, what is the required wash out period before changing to another drug with a different mechanism of action?15. When and how should changes between TNF, to or from another mechanism of action occur?16. What are the adverse events that should be considered when administering an anti-TNF?17. What should be the therapeutic approach in patients with hepatitis B who are receiving anti-TNF therapy?18. What should the therapeutic approach be in patients with TB who are receiving anti-TNF therapy?19. What should the therapeutic approach be in patients with HIV who are receiving anti-TNF therapy?20. What is the risk of malignancies in patients with RA treated with anti-TNF?21. What is the role of certolizumab pegol in the treatment of RA?22. What is the role of golimumab in the treatment of RA? |
| Treatment with rituximab, abatacept and tocilizumab |
| 23. What is the role of rituximab, abatacept and the tocilizumab in the treatment of RA?24. Is there any evidence that rituximab/tocilizumab/abatacept can be used as first-line therapy or as a first biologic in case of failure to traditional DMARDs?25. Is there evidence that rituximab is/tocilizumab/abatacept have a lower risk of TB compared with other anti-TNF agents? |
| Jak inhibitor treatment |
| 26. Do the use of tofacitinib in the treatment of RA have any support? Has it demonstrated clinical and/or imaging benefit? |
| Safety Recommendations, pharmacovigilance and special cases |
| 27. If a pregnancy is programmed in a patient with RA, how far in advance is suspending MTX, other traditional DMARDs and biological therapy recommended?28. In those patients with disease activity during pregnancy, what traditional DMARDs can be recommended?29. What traditional DMARDs may be used during lactation in patients with active disease?30. Is there evidence that traditional DMARDs affect fertility?31. What immunizations with live attenuated agents or inert agents are recommended in patients with RA? Can they be applied during active disease?32. In patients with hepatitis B or C virus infection or HIV infection, which of the traditional and/or biological DMARDs is it recommended to use?33. In patients with infections which traditional and biologic DMARDs are indicated?34. Is it advisable to discontinue leflunomide, MTX and other traditional DMARDs and biological before elective surgery and, if so, when should the drug be reinstituted?35. With which biological therapies is scrutiny for latent TB recommended?36. In which patients should prophylactic treatment for TB be considered?37. When should treatment with biological agents in patients receiving prophylaxis for TB be started? |
8 key elements that rule the management of patients with RA are considered:
- a)
Early diagnosis.
- b)
Timely treatment.
- c)
Management by rheumatologist.
- d)
Treat to target (remission or low activity level).
- e)
Individualized.
- f)
Regular follow up.
- g)
Consider comorbidity.
- h)
Adapted to the realities of clinical practice.
The main objective of treatment in patients with RA is remission or at least achieving a low level of clinical activity. The obligatory target in all patients is to control disease activity in order to improve symptoms, reduce joint damage, prevent functional limitations and improve the quality of life [LE: 1, DR: A].6,16
3. What Is the Role of Analgesics, NSAIDs, Glucocorticoids and Neuromodulators in the Treatment of RA?NSAIDs are useful for treating pain and inflammation. However, they do not alter the course of disease [LE: 1 + +, DR: A].17
They are used at the beginning of the disease and should be prescribed in combination with DMARDs [LE: 1, DR: A].1 They can also be used in case of a relapse and the continued uncontrolled symptoms despite a good objective response to DMARDs. Glucocorticoids especially should be used as “bridging” therapy in relation to the time needed to reach effective therapeutic DMARD levels or during relapse [LE 4, DR: D].6
Patients at risk of developing NSAID-associated gastric ulcers should receive gastroprotection based on a proton pump inhibitor [LE: 2, DR: A].17
All NSAIDs should be used at high doses for at least a week before considering a treatment failure. Once symptoms are controlled, NSAID should be used at the lowest effective dose and for the shortest time necessary, as adverse events are dependent on the dose and duration of treatment [LE: 1 + +, DR: A].17
In patients who continue to have activity in a few joints, despite a good therapeutic response to DMARD regimen, slow release glucocorticoid intraarticular infiltration with sterile technique at any time of the disease, after excluding septic arthritis and during up to 3-4 times a year may be carried out [LE: 1, DR: A].18
Due to its side effects, its use should be reduced to the lowest possible dose and be administered for the shortest period of time, according to the disease activity [LE: 4, DR: D].18
When there is severe pain, no analgesic response to previous treatments and no surgical options, opioid analgesics may be administered. There is limited evidence of the effectiveness of weak oral opioids to serve as effective analgesics in patients with RA, and, unfortunately, adverse events occur frequently. There is insufficient evidence for the use of weak opioids for periods of time over 6 weeks or the potential role of strong opioids.
If the pain is neuropathic, antidepressants (amitriptyline or duloxetine) and some neuromodulators (gabapentin, pregabalin or carbamazepine) can be used.
4. Can You Coadminister an NSAID or Glucocorticoid With MTX?Concomitant use of NSAIDs and MTX is safe with proper follow up; the objective of co-administration is to achieve the therapeutic goal in the shortest possible time, and identify cases resistant to initial therapy as soon as possible.19
In recent onset RA, the use of low-dose prednisone in combination with DMARDs was associated with higher rates of clinical remission [LE: 1, DR: A].20
5. When Should Treatment With a DMARD Be Started?Once the diagnosis of RA is established, DMARDs should be initiated. In Mexico the diagnosis is not usually made early [DR: A].21
There is evidence that the response and disease progression are superior when a DMARD is started within the first 3 months since disease onset compared to disease progression when treatment begins after 12 months. Therefore, treatment with DMARDs should be started as soon as possible [LE 2 b, DR: B].19
6. What DMARDs Should Be Considered as a First Choice?MTX should be used as the first-line synthetic DMARDs because it improves functional capacity and reduces radiographic progression with a good safety profile [LE: 1, DR: A].9
When there is a contraindication to MTX, LEF or SSZ should be used [LE: 1 a, DR: A].21
LEF, compared with placebo in studies has shown a capacity to reduce disease activity and slow radiographic progression. Its effectiveness was observed to be comparable to MTX in a meta-analysis [LE: 1 +, DR: A].22
CLQ, SSZ and HCQ are drugs that should be considered as first-line treatments in mild forms of the disease or recent-onset RA without unfavorable prognostic factors (rheumatoid factor [RF] or positive anti-CCP) [LE 1, DR: A].19
Feedback From the PanelMTX and other DMARDs (CLQ, SSZ) are widely available in the public health systems in Mexico. The panel believes that the use of MTX as initial monotherapy can lead to success in a significant number of patients with sufficient feasibility.
The working group suggests that LEF can be recommended as first-line treatment in specific cases of patients with contraindications to MTX, especially in patients with severe extra-articular manifestations, or as second line treatment in case of lack of response to MTX therapy or combined therapy with this remission-inducing drug. Liver and gastrointestinal toxicity should be monitored.
Other DMARDs such as SSZ and antimalarial drugs may be considered in women who wish to plan a pregnancy.
In patients infected with hepatitis B, C or HIV the use of sulfasalazine or antimalarials can be considered.
7. What Studies Are Required Before Starting Treatment With MTX and During Follow-up?Before the start of MTX a CBC, LFT, RFT and chest X-ray should be performed, as well as screening tests for hepatitis B, C and HIV in high-risk patients [LE: 2, DR: B].9 Post-treatment monitoring should include CBC [LE: 2],9 LFT [LE: 1]9 and RFT [LE: 2].9 CBC and LFTs should be monitored every 4 weeks during the time of dose adjustment, then every 8-12 weeks. The MTX dose should be decreased if a transaminase elevation above 2-3 times its normal value is detected and suspended in case this measure does not achieve a reduction thereof. In rare cases, a liver biopsy should be considered if there is persistent elevation of aminotransferases above 2-3 times ULN which is not attributable to other causes, despite the suspension of MTX. It is important to rule out pregnancy before starting MTX. Patients of childbearing potential and sexually active should be informed of the potential risks during pregnancy [LE: 4, DR: D].19
8. Is There Evidence in the Literature That Parenteral Administration of MTX Is More Effective Than the Oral Administration?Because of its increased bioavailability, the administration of intravenous MTX is more efficient than oral administration. We suggest considering the change to parenteral administration in cases of apparently inadequate response or gastrointestinal toxicity.19
9. Is It Safe to Use MTX in Patients With Interstitial Lung Disease?The presence of chronic lung disease is a relative contraindication to treatment with MTX so, if identified, interstitial lung disease in patients with RA who are receiving MTX should be considered as a motive to switch to another DMARD such as LEF, CLQ, HCQ, SSZ or a biological agent, either singly or in combination, according to individual patient context.23
10. When Should Traditional DMARDs Be Combined?Combination therapy with DMARDs should be considered in the following situations: in patients with severe active early to moderate disease, subsequent persistence of symptoms at 3 months after starting DMARDs [LE: 1 + +, DR: A]21 and in the presence of several factors of poor prognosis.21
Combination therapy with DMARDs should include MTX as an axis. MTX combination therapy with LEF, CLQ or HCQ and/or SSZ has proven effective in reducing the signs and symptoms of active disease in cases of inadequate response to monotherapy.6
Combination therapy with LEF+MTX may be a therapeutic option for patients with persistent joint activity despite the use of MTX as monotherapy. There is evidence showing superior efficacy of LEF + MTX compared to MTX + placebo [LE: 1 + +, DR: A].24
The evidence in clinical practice for the permanence of the combination LEF+MTX has been compared with the use of LEF as monotherapy, and it has shown that about 65% of patients remain on the combination of LEF+MTX at 30 months, similar to LEF monotherapy, where 55% continued treatment at this time. In this same study, the adverse effects forced 15% of patients to abandon the treatment, which represents a significant, but not higher than what is seen with monotherapy, expected rate [LE: 2, DR: B].25
A meta-analysis demonstrated a higher rate of treatment discontinuation, including due to drug toxicity, in RA patients receiving combination therapy with synthetic DMARDs than those with DMARD monotherapy. The most commonly used combination therapy was MTX+SSZ/antimalarials.26
Feedback From the PanelCombination therapy with synthetic DMARDs is a therapeutic strategy widely used in Mexico and has a higher frequency of use than biological agents, as it is widely available and it has a lower cost for public health institutions. As clinical experience of the working group, between 50 and 70% of patients with RA undergo, at some point in their evolution, combination therapy with synthetic DMARDs.
The Working Group believes that the combination of 2 or more of the following factors were associated with a poor prognosis: persistent moderate to severe activity of the disease after 3 months with monotherapy, failing a second DMARD monotherapy scheme, early erosions in less than one year since disease onset, high titers of rheumatoid factor or positive anti-CCP, or some extra-articular manifestations such as interstitial lung disease or rheumatoid vasculitis.
The working group recommended that, although there is evidence to support the efficacy of the combination MTX + LEF in patients with inadequate response to MTX, it is recommended that this combination be considered mainly in patients with moderate to severe activity who have failed MTX. Gastrointestinal and liver toxicity should be monitored. When this combination therapy is prescribed, a reduction in the dose of one of the 2 DMARDs and concomitant administration of folic acid should be considered.
Barriers to implementation. There are no barriers to its implementation. Most synthetic DMARDs are available in public sector hospitals. It is advisable to monitor the toxicity involved in combination therapy with synthetic DMARDs.
11. Is There Evidence in the Literature to Pass From DMARD Combination Therapy to Monotherapy Once the Therapeutic Response Has Been Achieved?The general recommendation is to use the least possible number of DMARDs in order to obtain a significant improvement of the disease and, once obtained, to try spacing the dose of one of the DMARDs until its removal.
12. What Is the Role of Cyclophosphamide in the Treatment of RA?It is used in patients with severe extra-articular manifestations such as interstitial pneumonitis or rheumatoid vasculitis. It is not recommended to treat joint or synovitis activity due to its high toxicity profile [LE: 1, DR: A].27
13. What Is the Role of Anti-TNF Treatment in RA?They are indicated in patients who had failure or intolerance to DMARDs including a biologic [LE: 1, DR: A].6 Anti-TNF therapy should be continued only if there is response at 6 months [LE: 2, DR: B].18
14. In Patients With Active RA Who Are Using Anti-TNF, What Is the Wash-out Period Required Before Changing to Another of a Different Mechanism of Action?The evidence for this recommendation is poor. Regarding the wash-out period, the clinician should individualize the decision in cases of lack of response of an anti-TNF, before starting another biologic with a different mechanism of action.
15. When and How do Changes Between TNF to or From Another Mechanism of Action Have to Be Made?Anti-TNF therapy should be continued only if there is an adequate response at 6 months [LE: 2, DR: B].18 In patients who have failed an anti-TNF due to lack of efficacy or toxicity at 6 months, a switch to another anti-TNF can be made [LE: 1, II],6 a switch to another biologic with a different mechanism of action, such as RTX TCZ and abatacept [LE: 1],6 or MTX or another DMARD can be added in case the anti-TNF has been administered as initial monotherapy [LE: 1].6
The possibility of a good response to a second anti-TNF is better when the first anti-TNF was suspended due to side effects and inefficacy [LE: 2 +, DR: C].18
Increasing the dose of anti-TNF is not recommended [LE: 1, DR: A].18
Treatment with RTX is recommended as an option in cases of inadequate response, intolerance or failure to DMARDs, including at least one anti-TNF [LE: 2, DR: B].28 However, RTX may not be effective in all patients with RA. At least one trial suggests that patients who are seropositive for rheumatoid factor and anti-CCP antibodies are those which respond best, while seronegative do not respond so clearly. Seropositivity should be evaluated in patients in whom this change is being considered.
Adalimumab, etanercept, infliximab and abatacept, in combination with MTX, are recommended in cases of inadequate response, intolerance or failure to DMARDs, including at least one biological, and who cannot receive RTX or this must be removed due to adverse effects [LE: 2, DR: B].29
The anti-TNF drugs adalimumab, etanercept, infliximab, certolizumab pegol and golimumab in combination with MTX, are recommended as a therapeutic option in patients with severe activity, DAS28 greater than 5.1 and who have presented failure to conventional DMARDs, including MTX [LE: 2, DR: B].30
16. What Are the Adverse Events That Should Be Considered When Administering an Anti-TNF?There is a 2-fold increase in the risk of developing infections with the use of anti-TNF. The most common sites of infection are the respiratory tract, bones and joints, urinary tract and skin [LE: 1, DR: A].31
Adverse events were more frequent in the treatment group receiving biological drugs, especially in reactivation of latent TB (OR: 4.68, CI 95%: 1.18–18.6) compared with the control group. Certolizumab has been associated with an increased risk of serious infections (OR: 2.04, 95% CI 1.43–2.91) compared with other anti-TNFs. Abatacept, adalimumab, etanercept and golimumab demonstrated fewer adverse events than infliximab [LE: 1 + +, DR: A].32
With regard to TB (considered as a positive PPD or tuberculin test with induration of 5mm or more), available data suggest a stronger risk of reactivation of TB with infliximab than with etanercept [LE: 3].7
The reactivation of herpes zoster virus is common in patients during treatment with anti-TNF. Few cases of varicella zoster have been documented [LE: 2, DR: B].7
17. What Should Be the Therapeutic Approach in Patients With Hepatitis B who Are Receiving Anti-TNF therapy?In patients with hepatitis B virus infection, treatment with an anti-TNF should be considered in selected cases receiving antiviral treatment and always in consultation with the hepatologist. The treatment should be started 2 weeks before the biological drug and continued for 6–12 months 7.
18. What Should Be the Therapeutic Approach in Patients With TB Who Are Receiving Anti-TNF Therapy?TB prophylaxis should be initiated at least 3 weeks before the start of anti-TNF [LE: 2, DR: B].6 Treatment consists of rifampicin 10mg/kg/day plus isoniazid 3–5mg/kg/day for 3 months, once a day or, as an alternative scheme, isoniazid 3–5mg/kg/day for 9 months in patients with intolerance to rifampicin, hepatic cirrhosis or in elderly patients [LE: 1, DR: A].6
In patients with active TB the full treatment with rifampicin, isoniazid, ethambutol and pyrazinamide should be given from 6 to 18 months, and the anti-TNF not started until completion of this scheme [LE: 2, DR: B].6 In these patients, only if the benefit outweighs the risk can the anti-TNF be restarted after 2 months of presenting clinical, radiographic, and serologic evidence of disease resolution [LE: 2, DR: B].6
19. What Should Be the Therapeutic Approach in Patients With HIV who Are Receiving Anti-TNF Therapy?HIV infection is a relative contraindication for the use of all biological products. However, given the new antiretroviral therapies, they should be considered for use in patients with disabling disease and without the possibility of receiving other treatments.33
20. What is the Risk of Neoplasia in Patients With RA Treated With Anti-TNF?The information regarding the risk of cancer in patients with RA treated with TNF inhibitors is highly variable and is biased by factors that include the underlying disease itself, which increases the risk of some forms of cancer (mainly lymphomas, more associated with serious disease and high inflammatory activity), the high degree of disease activity, to the dose of treatment used, to the other treatments used to control the disease (which may also increase the risk of cancer) and factors related to each individual (genetic predisposition). In the Spanish Registry of Adverse Events of Biological Therapies in Rheumatic Diseases (BIOBADASER) there was no association found between the use of anti-TNF and the occurrence of lymphoma.
In a series of 26 cases (18 with etanercept, 8 with infliximab) there was a higher prevalence of non-Hodgkin lymphomas observed. The interval between start of treatment and diagnosis of lymphoma was very short (average of 8 weeks) and 2 patients (one with each drug) had lymphoma regression after discontinuation. Two patients previously treated for lymphoma and who were in remission at the start of anti-TNF therapy, quickly developed a recurrence.34
The association between anti-TNF and solid tumors is lower, although there seems to be an increased risk in relation to basal cell skin tumors (but not melanoma).35 Recently six cases of hepatosplenic lymphoma with a very aggressive course have been reported in young patients with Crohn's disease treated with infliximab as well as with ASA or 6-mercaptopurine (Centocor communication).
Follow up data are required in the longer term, as well as a greater number of patients to clarify the existence of an association of TNF inhibitors in the development of tumors. Meanwhile, caution should be taken to indicate the use of these drugs when there is a history of previous tumors.
21. What is the Role of Certolizumab in the Treatment of RA?Certolizumab reduces the symptoms of the disease, slows radiographic progression, improves quality of life and functional capacity in patients with moderate to severe RA, and can be used alone or in combination with MTX in patients without prior treatment with DMARDs, and those refractory to MTX and anti-TNF [LE: 2 +, DR: D].36
22. What is the Role of Golimumab in the Treatment of RA?Golimumab reduces the symptoms of the disease, slows radiographic progression in RA patients with moderate to severe disease, and can be used as monotherapy or in combination with MTX in patients without prior treatment with DMARDs and refractory to MTX and anti-TNF [LE: 2 +, DR: A].37
Implementation barriers. The anti-TNF etanercept, infliximab, adalimumab and certolizumab are very expensive drugs and which are not available in all health institutions; only the Mexican Social Security Institute (IMSS), the Institute of Social Security and Services for the Government Workers (ISSSTE) and Petroleos Mexicanos (PEMEX) hospitals have them. To the date of the preparation of this document golimumab is not available in Mexico.
23. What is the Role of Rituximab, Abatacept and Tocilizumab in the Treatment of RA?They are recommended for patients who had an inadequate response to treatment with DMARDs or anti-TNF [LE: 1, DR: A].6 They are also recommended in patients who have not responded to treatment with 2 anti-TNF agents [LE: 2, DR: C].
In the case of rituximab, clinical response is better in patients with positive rheumatoid factor (RF) and/or anti-CCP [LE: 1, DR: A] antibodies.6
24. Is There Any Evidence That Rituximab/Tocilizumab/Abatacept Can Be Used as First-line Therapy or as a First Biologic in Case of Treatment Failure to Traditional DMARDs?Generally, their use is not recommended as first-line treatment. However, abatacept itself could be used as a first-line in combination with MTX, according to the results of the AGREE study38 and the post hoc analysis of data from that study.39 However, our group does not recommend the use of these three drugs as first-line without having tried MTX first.
With regard to their use as a first biologic agent, it has already been mentioned that the 3 agents are recommended in patients who had an inadequate response to DMARD therapy.6 Rituximab may be used before an anti-TNF agent, especially in patients who have a relative or absolute contraindication for this type of drug, according to the guidelinelines of the British Society of Rheumatology,40 or in some special situations such as a history of B cell lymphoma, or the presence of latent TB, multiple sclerosis, vasculitis or concomitant overlap syndromes, as recommended by the guidelinelines of the Canadian Rheumatology Association [LE: 1, DR: A].6
Implementation barriers. Rituximab, tocilizumab and abatacept are very expensive drugs and not available in all health institutions. In some cases special administrative procedures are required to obtain them.
25. Is There Evidence That Establishes That Rituximab/Tocilizumab/Abatacept Have a Lower Risk of TB Compared With Anti-TNF Agents?There are no reports of an increased risk of TB in patients treated with rituximab for lymphoma. The risk regarding the use of tocilizumab and abatacept is unknown [LE: 4, DR: B].7
26. Does the Use of Tofacitinib in the Treatment of RA Have Support? Has It Demonstrated Clinical and/or Imaging Benefit?Tofacitinib has demonstrated clinical and radiographic benefit in patients with active RA who have failed at least one DMARD [LE: 2 +, DR: C].41
Studies show higher ACR 20, 50, 70 responses, increased frequency of DAS28 remission and better functionality in patients receiving tofacitinib than in those receiving placebo [LE: 2 +, DR: C].42
A close eye should be kept on the decrease in neutrophil and in lymphocyte counts, hemoglobin levels, and increases in blood lipid. Recently, long term studies following tofacitinib-treated patients have warned of the presence of serious and difficult to manage complications (opportunistic infections) as well as certain tumors, hepatotoxicity, gastrointestinal perforation and infection. Therefore, the European Drug Regulatory Agency (EMA) has denied approval for to facitinib marketing in Europe and the Food and Drug Administration (FDA) has issued an international alert, recommending that patients currently involved in long term studies continue to receive the medicine.43,44
Implementation barriers. It should be noted that data from long-term monitoring are limited, it is expected to have a high cost (similar to anti-TNF), and as with other DMARDs, an increased risk of serious infections, including TB, fungal infections and infections generated by opportunistic pathogens, so patients receiving this drug must be closely monitored.43
27. If a Pregnancy Is Programmed in a Patient With RA, how Far in Advance Is Suspending MTX, Other Traditional DMARDs and Biological Therapy Recommended?Methotrexate. During pregnancy the use of MTX is formally contraindicated. It should be discontinued preferably 4 months before pregnancy, and in case of lack of planning, it should be discontinued immediately [LE: 4, DR: D].45
Leflunomide. Women treated with LEF must wait 2 years after suspending it to schedule a pregnancy. In pregnant women who wish to get pregnant before 2 years, we recommend a wash out with cholestyramine 8 every/8h, or activated charcoal 50g every 6h for 11 days (in both cases), thus achieving elimination of the drug in 3 months. After washing, it is desirable to quantify plasma levels of the active metabolite, which must be less than 0.02mg/l. This level will be verified 14 days after the first determination [LE: 1, DR: A].45
A case series (n=45) showed 2 products of pregnancy with congenital malformations when exposure occurred in the first trimester (n=16) and no malformation in patients who discontinued treatment before conception (n=29).[LE: 4, DR: D].46
Anti-TNF. Therapy with anti-TNF is contraindicated in pregnancy. The interval the patient should be free of anti-TNF to conceive, with infliximab is 6 months, with adalimumab, 5 months and with etanercept without recommendation [LE: 3, DR: C].10 In the event of a pregnancy in women with RA under biological treatment, this treatment should be discontinued immediately [LE: 4, DR: C].45
Some published recommendations suggest that the safety interval between the last dose of anti-TNF and conception is 5 half-lives of the drug.
28. In Those Patients With Disease Activity During Pregnancy, what Traditional DMARDs Can Be Recommended?Not all patients with RA improve during pregnancy. Both HCQ and CLQ 47, azathioprine45 and SSZ47 are safe pharmacological interventions in patients with RA disease activity during pregnancy.
Azathioprine is recommended with caution if necessary during pregnancy, to suppress the activity of RA [LE: 4, DR: D].45
29. What Traditional DMARDs May Be Used During Lactation in Patients With Active Disease?CLQ and HCQ are safe pharmacological treatments during lactation in patients with RA [LE: 4, DR: D].45 SSZ can be used with caution while breastfeeding. Its use is not recommended if the infants renal function is affected [LE: 4, DR: D].48
The British guidelines mention discontinuing infliximab 6 months before initiating breastfeeding, with the same indication for adalimumab and etanercept [LE: 2, DR: B].10
30. Is there evidence that a traditional DMARD affects fertility?In women there are no reports. However, in men, SSZ generates reversible oligospermia.
31. What Immunizations With Live Attenuated Agents or Inert Agents Are Recommended in Patients With RA? Can They Be Applied During Active Disease?Evaluation of the vaccination status of the patient is recommended during the initial evaluation of patients with inflammatory rheumatic diseases. Ideally, vaccination should be administered during the stable phase of the disease [LE: 4, DR: D].49 Vaccination recommendations should be reviewed by age group and gender.
Barriers to implementation: none.
Immunization with live attenuated microorganisms should be avoided whenever possible in immunosuppressed patients with autoimmune inflammatory rheumatic diseases [LE: 4, DR: D].49
Barriers to implementation: none.
When it is necessary to administer an immunization with live attenuated microorganisms (measles, mumps, rubella, typhoid, polio [oral]), it is suggested it be given 2 weeks before and ideally four weeks before the onset of treatment with DMARDs or biological drugs [LE 4, DR: D].7
Barriers to implementation: none.
Vaccination against influenza and pneumococcal disease in RA patients before or during treatment with DMARDs or biological drugs is recommended. Pneumococcal vaccine should be reapplied every 5 years [LE: 2, DR: B].7,45,49
Barriers to implementation: none.
In patients who have not been immunized against pneumococcus and prior to the start of RTX, the administration of pneumococcal polysaccharide 23-valent vaccine, with 4-6 of anticipation is recommended [LE: 2, DR: B].40
Barriers to implementation: none.
It is recommended that the influenza vaccine be administered before the start of RTX and annually, preferably before retreatment [LE: 2, DR: B].40
Barriers to implementation: none.
Patients with autoimmune inflammatory rheumatic diseases should be vaccinated with tetanus toxoid, in accordance with established recommendations for the general population [LE: 4, DR: D].4,40
Barriers to implementation: none.
The administration of the vaccine against herpes zoster is recommended in patients receiving MTX at doses ≤25mg/week and/or glucocorticoids at doses <20mg/day [LE: 4, DR: D].7
Barriers to implementation: vaccine not available in Mexico.
The ideal time for its application would be between 2 and 4 weeks before the onset of biological treatment.
The vaccine against herpes zoster should be considered in patients with RA over 60 years of age [LE: 4, DR: D].7
Barriers to implementation: depends on the availability of the vaccine in Mexico.
Vaccination against hepatitis B should also be carried out.
32. In Patients Infected With the Hepatitis B, C or HIV Virus, Which of Traditional DMARDs and/or Biological Are Advisable for Use?Prior to initiation of treatment with biological agents, it is recommended to investigate the possibility of infection with HIV and hepatitis viruses in patients with risk factors [LE: 4, DR: D].40,50,51
In patients infected with hepatitis C (active or inactive), anti-TNFs appear to be safe. However, it should be used with caution if any evidence of viral replication is present.
The use of biopharmaceuticals in patients with viral infections should be avoided. In patients under treatment, the development of viral infections should be carefully monitored. If present, suspension of the biological should be carried out and the patient should receive specific treatment according to the type and site of infection [LE: 1, DR: A].51
When suspecting varicella infection in patients receiving biological agents, treatment should be discontinued and specific antiviral treatment indicated immediately [LE: 2, DR: B].45
33. In Patients With Infections, Which Traditional and Biologic DMARDs Are Indicated?The safety profile of the TNF inhibitors is similar. The Biobadamex 1.0 Mexican Registry identified an increased risk for infections (RR 2.05, 95% CI: 1.5–2.7, P<.001) in subjects under biological therapy vs those receiving traditional DMARDs. In patients with infections, the use of agents anti-TNF drugs should be avoided [LE: 2, DR: C].7,31,52,53
34. Is It Advisable to discontinue leflunomide, MTX and Other Traditional and Biologic DMARDs Before Elective Surgery and, if so, when Should the Drug Be Reinstituted?Yes it is recommended, since treatment with traditional and biologic DMARDs is immunosuppressive, there is an increased risk of infectious complications surrounding a surgical procedure. The revised guidelinelines summarize the evidence on the safety of treatment with MTX in elective surgeries, as well as the suspension a week before surgery.
As to the suspension of biological therapy, this will depend on the individual circumstances of each patient and the nature of the surgery and the half-life of drugs. The evidence underpinning the Canadian guidelinelines mentions that 1-2 months before surgery anti-TNF should be discontinued. As for RTX, time is defined more by the amount of B cells and in situations where the disease is well controlled.
The restoration of a biologic drug depends on the clinical setting (RA activity and postoperative course free of infection) [LE: 4, DR: B].45,51
35. In Which Biological Therapies for Latent TB Is Scrutiny Recommended?For all patients undergoing biological treatment a complete history, Combe, intradermal testing and chest X-ray should be performed. Another test used for latent TB, called IGRA (INF γ release assays), evaluates the in vitro production of IFN in the presence of Mycobacterium tuberculosis-specific antigens by enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunosorbent Spot assay (ELISPOT), demonstrating a greater sensitivity and specificity than tuberculin, especially in patients who have received the Bacillus Calmette-Guérin (BCG) vaccine.54
Barriers to implementation: none for traditional screening, but IGRA is not readily available in Mexico.
36. Which Patients Should Be Considered for TB Prophylactic Treatment?All patients presenting a PPD equal or greater than 5mm, whether or not they have been vaccinated with BCG or have a positive Combe with a chest X-ray suggestive of TB.
Feedback From the PanelBecause of the prevalence of TB in Mexico, when there is a high suspicion of disease, even with negativity of the above tests, QuantiFERON testing is recommended, if the resource is available [LE: 4, DR: B].6,45,50
Barriers to implementation: limited access to IGRA.
37. When Should Treatment With Biological Agents in Patients Receiving Prophylaxis for TB Be Started?This is not clearly defined. It is recommended that one to two months pass before starting biological agents or, starting at the same time, if the case merits it [LE: 4, DR: D].50
Feedback From the PanelWe recommend starting with anti-TNF 3-4 weeks after starting prophylaxis [DR: D].
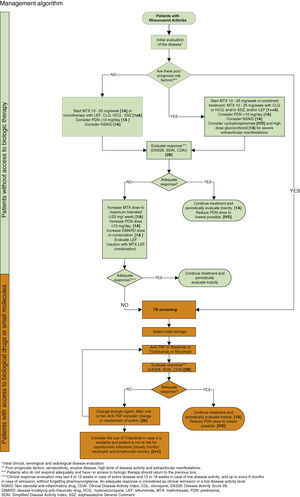
Management AlgorithmGeneral Comment- •
The safety recommendations of pharmacotherapy in daily clinical practice are limited and related to the Mexican population.
- •
It is advisable to strengthen methods of passive pharmacovigilance (adverse event reports) and active pharmacovigilance (cohort studies, registries, pharmacoepidemiological designs) targeting risk estimation and decision making for the benefit of the patient.
- •
For reporting adverse drug reactions (case report) to the health authority (NOM-220-SSA1-2002) data should include: relevant patient information (age, gender, hepatic and renal function, weight/height in pediatric population, allergies, etc.), details of the drug causing the adverse reaction (dose, dosing interval, concomitant pharmacotherapy, batch, expiry date and manufacturer [useful for determining differences in quality between producers]), the description of the adverse reaction (type, treatment employed, consequences in the patient and in compliance).
- •
The Mexican College of Rheumatology supports the implementation of pharmacovigilance activities to provide data generated in our population in order to prevent or mitigate risks related to pharmacotherapy in patients with RA.
The authors state that no experiments were performed on persons or animals for this study.
Data confidentialityThe authors state that they have followed their workplace protocols regarding the publication of patient data and all patients included in the study have received enough information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors state that they have obtained informed consent from patients and/or subjects referred to in this article. This document is in the possession of the corresponding author.
FinancingThe Mexican College of Rheumatology received an unrestricted educational grant from SANOFI.
The staff working at SANOFI had no role with the management of information contained in this manuscript or participated in the meetings of the working group.
Conflict of InterestDr. Mario H. Cardiel: principal investigator in clinical trials with Roche, Bristol-Myers Squibb, Pfizer, Astellas, Anthera, Astra Zeneca, Lilly, Amgen, La Jolla Pharmaceutical Company. Advisor to Pfizer, Lilly, MSD, Amgen.
Dr. Alejandro Diaz Borjón: UCB lecturer.
Dr. Monica Espinosa Vázquez del Mercado: none.
Dr. Jorge Ivan Gamez-Nava: none.
Dr. Leonor A. Barile Fabris: lecturer Roche, Pfizer, UCB, Novartis, GSK.
Dr. Cesar Pacheco Tena: lecturer Roche, BMS, Janssen, Abbott (Abvie), UCB and Pfizer.
Dr. Luis H. Silveira Torre: principal investigator in clinical trials with Roche and Bristol-Myers Squibb.
Dr. Virginia Pascual Ramos: none.
Dr. Maria Victoria Goycochea Robles: none.
Dr. Enrique Aguilar Jorge Arreola: none.
Dr. Verónica González Díaz: none.
Dr. Jose Alvarez Nemegyei: UCB consultant.
Dr. Laura del Carmen González-López: none.
Dr. Mario Salazar Páramo: none.
Dr. Margarita Hernández Portela: lecturer MSD, Roche, Pfizer, UCB, Janssen, Grünenthal.
Dr. Zully Castro Colin: none.
Dr. Daniel Xavier Xibillé Friedman: Pfizer lecturer and clinical trials with Pfizer, AztraZeneca, Celltrion.
Dr. Everardo Alvarez Hernández: none.
Dr. Mario Salazar Páramo: none.
Dr. Julio Casasola Vargas: none.
Dr. Miguel Cortés Hernández: none.
Dr. Diana E. Flores Alvarado: AztraZeneca and UCB lecturer. Subinvestigator for Quintiles.
Dr. Laura Aline Martinez Martinez: none.
Dr. David Vega Morales: none.
Dr. Luis Felipe Flores-Suarez: none.
Dr. Gabriel Medrano Ramirez: none.
Dr. Antonio Barrera Cruz: none.
Dr. Adolfo García González: none.
Dr. Maricela Susana López López: none.
Dr. Alejandra Rosete Reyes: none.
Dr. Rolando Espinosa Morales: lecturer Lilly and Sanofi.
The authors wish to thank Dr. Eduardo Barreira Mercado, President of the Mexican College of Rheumatology, for providing the logistical support needed to complete this collegiate initiative. They would also like to thank Dr. Jorge Aldrete and Dr. Ana Cantú for the support in the editorial work on this manuscript.
Please cite this article as: Cardiel MH, Díaz-Borjón A, Vázquez del Mercado Espinosa M, Gámez-Nava JI, Barile Fabris LA, Pacheco Tena C, et al. Actualización de la Guía Mexicana para el Tratamiento Farmacológico de la Artritis Reumatoide del Colegio Mexicano de Reumatología. Reumatol Clin. 2014;10:227–240.