To analyse the efficacy, adherence, patient satisfaction, safety, pharmacodynamics and cost-effectiveness of parenteral methotrexate (MTX) in patients with rheumatic diseases.
MethodsA systematic review of literature was carried out in Medline, Embase and Cochrane Central from the beginning until June 2019. Studies including adult patients with rheumatic diseases being treated with parenteral MTX were identified and data on efficacy, adherence, satisfaction, safety, pharmacokinetics, and cost-effectiveness analysed. As for the designs, systematic reviews, clinical trials, or observational studies were permitted, including cross-sectional and small-sample studies if they were pharmacokinetic studies.
ResultsOut of 4160 identified articles, 80 articles were finally included. The efficacy profile of parenteral MTX seems useful in general and in those patients with insufficient response to oral MTX. The parenteral route does not seem to increase the rate or severity of adverse events due to the use of MTX. The use of parenteral MTX is an appropriate way to reduce costs in patients with inadequate response to oral MTX. Adherence and satisfaction are favoured by training programmes in the use of the parenteral route. The results in rheumatic diseases other than rheumatoid arthritis (RA) are very scarce and do not enable obtaining conclusive data.
ConclusionsParenteral MTX can be an alternative to the use of oral MTX, due to its profile of efficacy, safety, adherence and pharmacoeconomic results, especially in those patients with RA.
Analizar la eficacia, adherencia, satisfacción del paciente, seguridad, farmacodinámica y costo-efectividad del (MTX) parenteral en pacientes con enfermedades reumáticas.
MétodosSe llevó a cabo una revisión sistemática basada en una estrategia de búsqueda en Medline, Embase y Cochrane Library (inicio-06/2019). Se identificaron estudios que incluyeran pacientes adultos con enfermedades reumáticas en tratamiento con MTX parenteral y que analizaran datos de eficacia, adherencia, satisfacción, seguridad, farmacocinética o costo-efectividad. En cuanto a los diseños se permitieron revisiones sistemáticas, ensayos clínicos o estudios observacionales, incluyendo transversales y estudios con muestras pequeñas si eran estudios de farmacocinética.
ResultadosDe 4.160 artículos identificados, se incluyeron finalmente 80. El MTX parenteral parece útil de manera general y en especial en aquellos pacientes con respuesta insuficiente a MTX oral. La vía parenteral no parece aumentar la tasa ni la gravedad de los eventos adversos con respecto a la oral y podría reducir costes en aquellos pacientes con respuesta inadecuada a MTX oral. La adherencia y satisfacción se ven favorecidos por programas de entrenamiento en la vía parenteral. Los resultados en enfermedades reumáticas distintas a la artritis reumatoide (AR), son muy escasos y no permiten obtener datos concluyentes.
ConclusionesEl MTX por vía parenteral podría ser una alternativa al uso de MTX oral, por su perfil de eficacia, seguridad, adherencia, satisfacción y resultados fármaco-económicos, especialmente en pacientes con AR.
Methotrexate (MTX) is currently the most widely used disease-modifying antirheumatic drug (DMARD) in rheumatology because of its effectiveness in symptomatic control, delayed joint damage, low cost, and favourable safety profile.
MTX is a structural analogue of folic acid that acts by competitively inhibiting the enzyme dihydrofolate reductase (DHFR) that is involved in the formation of folic acid, which, in turn, is necessary to create nucleoside thymidine, required for the synthesis of DNA, RNA, thymidylate, and protein.1 With this in mind, it is expected to be distributed primarily in organs containing high levels of DHFR (lung, liver, kidney, and gastrointestinal tract) and high cell turnover (skin, germ, and tumour cells), which accounts for its indications and main side effects. MTX acts by partially inhibiting the immune system and reducing long-term autoimmune joint inflammation.2
While its safety profile is well-established and advantageous, many patients can experience gastrointestinal intolerance, particularly with increasing doses, which may limit both its efficacy and even its use.3 Similarly, adherence to MTX treatment can also vary and is probably multifactorial (adverse events, non-adherence to chronic medications, etc.); in some studies, it has been found to be as high as 30%–40% of patients.4,5
Parenteral administration of MTX can be a useful alternative in many of these patients. The aim of this systematic review is to establish the profile of use, adherence, safety, satisfaction, pharmacokinetics, and pharmaco-economic analysis of parenteral MTX in patients with rheumatic diseases, based on the best available evidence.
Material and methodsA systematic review was carried out. To this end, studies were selected that included (1) adult subjects with rheumatic diseases, (2) in treatment with parenteral MTX, regardless of the route of administration, and that (3) analysed data on efficacy, safety, adherence, satisfaction, pharmacokinetics, or cost-effectiveness. As for design, studies with the following designs were accepted: systematic reviews, clinical trials, or observational studies with small samples, as long as they included pharmacokinetics. Animal and basic science studies were excluded.
Search strategySearch strategies were generated by an expert documentalist (MPR) with MeSH terms and free text in the following bibliographic databases: Medline (via OVID), Embase, and Cochrane Library, all from their inception until June 2019. Articles about studies in human subjects and in English or Spanish were used as limits. Subsequently, a secondary search of the bibliography of the articles finally included was performed.
Annex 1 shows the Medline search strategy. Based on this, Embase and Cochrane were generated. All citations resulting from the searches were entered into EndNote® libraries to facilitate their handling.
Article selection, data collection, and bias assessmentTwo reviewers, rheumatologists with extensive experience in systematic reviews (TO, EL), independently screened the articles resulting from the search strategy in the different bibliographic databases. In case of discrepancies, a third researcher (LC) was included. The search results were first screened by title and abstract, or by full article, for those lacking an abstract, in sessions lasting a maximum of 60min. Following this process, the full text of the selected articles was retrieved and analysed in detail. The articles retrieved were classified according to the topics of interest they covered (efficacy, safety, adherence, satisfaction, etc.) and the information was collected directly in tables prepared for this purpose. The Oxford Centre for Evidence-Based Medicine guidelines were used to homogeneously evaluate the studies, assigning a level of evidence to each study according to the question it answered.6
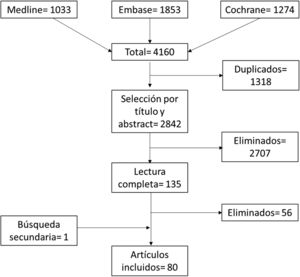
ResultsFig. 1 shows the flow chart of the article selection process. Of the 4,160 initially captured by the searches, and after eliminating duplicates and selection by title and abstract and full reading, 80 articles were finally included (full reading and excluded articles can be found in Appendix 1). Of these articles, 72 included participants with rheumatoid arthritis (RA), six with spondyloarthritis (SpA), three with systemic autoimmune diseases, and one with pyrophosphate deposition disease.
Table 1 presents the descriptive data of the included articles and Table 2 details their results, according to the topics of interest. The synthesis by questions and rheumatic diseases is presented below.
Table of evidence.
| # | Study | Duration | Population | Route admin. | Comparison (Before = > After) | Mean dose (mg/ wk) | Folic acid | Other ttm | Response variables | Quality |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ahmed 20107 | 24 wk | -RA | sc | sc vs. oral | 2mg/ wk w/ increasing doses | – | – | -Efficacy | 3b |
| CT | ||||||||||
| 2 | Arthur 19998 | – | n: RA=33; PsA=4 | im | vo--> im | 20mg/ wka | – | -Adhere | 2c | |
| O, R | −68% ♀ | -Satisfaction | ||||||||
| -age 49 | -Safety | |||||||||
| -DD 5 | ||||||||||
| 3 | Bakker 20109 | 3m | -n: RA=151 | sc | per os--> sc--> + cycles | Max 30mg/ wk | 0.5mg/d | -NSAID | -Efficacy | 2b |
| RCT | −77%♀ | |||||||||
| -age 54 | ||||||||||
| 4 | Branco 201610 | 7 yr | -n: RA=50 | sc | per os--> sc | 18.3mg/ wkb | – | -DMARDs | -Efficacy | 18.3mg/ wkb |
| O, R | −87% ♀ | -Biolog | -Safety | |||||||
| -age 55 | ||||||||||
| -DD 11yr | ||||||||||
| 5 | Bharadwaj 200711 | 12m | -n: RA=32 | sc | per os--> sc | 7.5-25mg/ wk | 15mg/ wk | -Efficacy | 3b-4 | |
| O, R | -age 61 | -Safety | ||||||||
| -DD 4yr | ||||||||||
| 6 | Bianchi 201812 | 12 wk | -n: RA=10 | sc | 50mg/ wk (for 4 wk, followed by 25mg /4 wk; and 15mg /4s) | 12mg/ wk | -Efficacy | 3a | ||
| Open-label CT | −70 % ♀ | -Safety | ||||||||
| -age 58 | ||||||||||
| 7 | Bingham 200313 | 24 wk | -n: RA=33 | im | per os--> im | Max 25mg/ wk | 5mg/ d × 6 d/ wk | -DMARDs | -Efficacy | 3b-4 |
| O, P | -age 51 | -Cortic | -Safety | |||||||
| 8 | Borman 201414 | 3m | -n: RA=80 | sc | per os--> sc | 16.5mg/ wk | – | -Cortic | -Efficacy | 3a |
| O,R | -age 54 | -NSAID | -Safety | |||||||
| -DD 122m | ||||||||||
| 9 | Braun 200815 | 6m | -n: RA=384 | sc | -per os--> sc | 15−20mg/ wkb | 5mg/ d | -Cortic | -Efficacy | 1b |
| RCT | −79% ♀ | -sc--> per os | -NSAID | -Safety | ||||||
| -age 58 | ||||||||||
| -DD 2.5m | ||||||||||
| 10 | Brooks 199016 | 1 wk | -n: RA=5 | im/ wkc | per os-->im-->sc | 24.5mg/ wk | -PK | 4 | ||
| O, P | −60% ♀ | |||||||||
| -age 45–75 | ||||||||||
| 11 | Burbage 200117 | 9m | -n: RA=24; SpA=4; SLE=2 | im | per os--> im | 10–15mg/ wk | – | -Efficacy | 3b-4 | |
| O,R | - Satisfaction | |||||||||
| -Safety | ||||||||||
| 12 | Calasan 201318 | – | -n: RA=249; PsA=42 | sc | per os vs. sc | 20.0mg/ wk | -NSAID | -Safety | 2b | |
| CS | −62.2% | -PIP | ||||||||
| -age 59.4 | -Cortic | |||||||||
| -DMARDs | ||||||||||
| 13 | Capone 200019 | 8 wk | -n: RA=29 | im | – | 7.5mg | – | -Cortic | -PK | 3b |
| O,P | −72.4%♀ | -Paracetamol | ||||||||
| 14 | Carpentier 199820 | 6m | -n: RA=23 | im | per os (before study initiation) | 10.7mg | 6 d/ wk | -Cortic | -PK | 2b |
| CT, crossover | −69.5% ♀ | -NSAID | ||||||||
| -age 61 | ||||||||||
| 15 | Chichasova 201821 | 36m | -n: RA=74 | sc | 15mg/ wk | 5–10mg/ wk | -NSAID | -Efficacy | 2b | |
| O,P | -age 45.2 | -Safety | ||||||||
| -DD 5.2m | ||||||||||
| 16 | Crespo 201422 | 5yr | Imaginary cohort | sc | sc vs. per os | -Econ | 2a | |||
| Ph-Ec | −78.9% ♀ | |||||||||
| -age 56 | ||||||||||
| 17 | Curtis 201623 | 12m | -n: RA=979 | sc | MTX sc vs. MTX per os vs. biolg | -Safety | 3b | |||
| O, P | −91% ♀ | |||||||||
| -age 48 | ||||||||||
| 18 | De Groot 199724 | – | -n: WG=17 | iv | – | 1.7–7.5mg/ day | -Cortic | -Efficacy | 3b-4 | |
| O, P | −47% ♀ | |||||||||
| -age 46 | ||||||||||
| 19 | Demary25 2014 | 6m | -n: RA=111 | sc | sc--> scc | 15–20mg/ wk | −1/ wk | -DMARDs | - Satisfaction | 2a |
| RCT | −73% ♀ | sc--> sc | -NSAID | -Safety | ||||||
| -age 54 – | Pt edu | |||||||||
| -DD 3 | ||||||||||
| 20 | Dhaon 201326 | 24 wk | -n: RA=66 | sc | 1) 7.5mg/ 2 or 3 times per wk | 15–22.5 | – | – | -Efficacy | 2b |
| CT | 2) 15–22.5mg/ oral | -Safety | ||||||||
| 3) 15–22.5mg/ iv | ||||||||||
| 21 | Dhaon 201727 | 24 wk | -n: RA=135 | im | per os/2 or 3 times / wk | 15–20mg/ wk | -HCQ | -Efficacy | 2a | |
| O, P | −80% ♀ | per os/ single dose | -Safety | |||||||
| -age 40 | ||||||||||
| -DD 67m | im | |||||||||
| 22 | Finckh 201428 | 3m | n: CPPD=26 | sc | sc | 15mg/ wk | 5–10mg/ wk | -Analg | -Efficacy | 3a |
| RCT, crossover | −56% ♀ | PBO | -Safety | |||||||
| -age 62 | ||||||||||
| 23 | Fitzpatrick 201129 | – | NICE | sc | -Econ | 3a | ||||
| Ph-Ec | ||||||||||
| 24 | Fitzpatrick 201130 | – | Costs in Great Britain | sc | -Econ | 3a | ||||
| Ph-Ec | ||||||||||
| 25 | Flipo 201831 | 6m | -n: RA=466 | sc | sc (with prior per os use) | 15.1mg | -Adhere | 3a | ||
| O, P | -age 59 | sc | ||||||||
| -DD: 6,5yr | ||||||||||
| 26 | Freundlich 201432 | 8 wk | -n: RA=101 | sc | per os--> sc | 1/25mg/ wk | – | -Satisfaction | 2a-b | |
| O, P | −79.2% ♀ | -Safety | ||||||||
| -age 60 | ||||||||||
| -DD 13yr | ||||||||||
| 27 | Godfrey 199833 | 36m | n: RA=62 | im | 10.4mg | – | -NSAID | PK | 2b | |
| −66% ♀ | ||||||||||
| -age 58 | ||||||||||
| -DD 153 wk | ||||||||||
| O, P | ||||||||||
| 28 | Gottheil 201634 | 3 yr | -n: RA=1,214 | sc | MTX per os vs. MTX sc vs. combined therapy | -Efficacy | 4 | |||
| −71.3% ♀ | ||||||||||
| -age 54 | ||||||||||
| -DD 5.5m | ||||||||||
| 29 | Gridneva 201535 | -n: RA=237 | sc | MTX per os vs. MTX sc vs. combined therapy | -Efficacy | 4 | ||||
| O, P | ||||||||||
| 30 | Gridneva 201535 | 12m | -n: RA=47 | sc | sc | Max 30mg/ wk | -Efficacy | 1b | ||
| O, P | −38% ♀ | |||||||||
| -age 51 | ||||||||||
| -DD 4.2m | ||||||||||
| 31 | Gridneva 201837 | 1 yr | -n: RA=106 | sc | 10–15mg/ wk (max 30mg) | Safety | 3b | |||
| O, P | ||||||||||
| 32 | Griffin 200438 | 6m | -n: RA=22 | sc/im | per os--> sc/im | 17.5mg/ wk | -Efficacy | 4 | ||
| O, R | ||||||||||
| 33 | Haibel 200739 | 16 wk | -n: EA=20 | sc | 15mg/ wk (4 wk)--> 20mg/ wk (12 wk) | -Cortic | -Efficacy | 2b | ||
| CT, open-label | −20% ♀ | -NSAID | -Safety | |||||||
| -age 40 | -DMARDs | |||||||||
| -DD 14yr | ||||||||||
| 34 | Hameed 201040 | 3m | -n: RA=103 | sc | per os --> sc | 15mg/ wk | – | -Efficacy | 4 | |
| O, R | −71% ♀ | |||||||||
| -age 55 | ||||||||||
| 35 | Hamilton 199741 | 18 m | -n: RA=21 | im | per os--> sc; MTX per os vs. MTX im | -Cortic | -PK | 3b | ||
| O, P | −67% ♀ | -NSAID | ||||||||
| -age 54 | ||||||||||
| 36 | Hammond 201542 | 8yr | -n: RA=49 | sc/ vo | MTX per os vs. MTX sc | 5-15mg/ wk | -Efficacy | 4 | ||
| O, R | -mean age 61 | |||||||||
| 37 | Harris 201843 | – | -n: RA=7.017 | -Efficacy | 3a | |||||
| O, R | −9.3% ♀ | -Safety | ||||||||
| -age 54 | ||||||||||
| -DD 5.2m | ||||||||||
| 38 | Hattesohl 201844 | 12 wk | -n: 478 (RA=39.3%; PsA=23.4%; Ps=23.4) | sc | sc vs. self-injector | Satisfaction | 3b | |||
| O, R | −57.1% ♀ | |||||||||
| 39 | Hazlewood 201645 | 12m | -n: RA=714 | sc | MTX per os vs. MTX sc | 22.3mg/ wk | – | -Cortic | -Efficacy | 2a |
| O, P | −75.1% ♀ | -DMARDs | -Safety | |||||||
| -mean age 54 | ||||||||||
| -DD 5,2m | ||||||||||
| 40 | Hoekstra 200446 | 2 wk | -n: RA=15 | sc | per os --> sc | -DMARDs | -PK | 3b | ||
| O, P | 73% ♀ | -Cortic | ||||||||
| -DD 7 yr | ||||||||||
| 41 | Huber_200647 | 11 m | -n: cutaneous LE=15 | sc | (previously MTX iv) | 7.5–20mg/ wk | 5mg 2 d/ wk | -HCQ | -Efficacy | 3b-4 |
| O, R | -Cortic | |||||||||
| 42 | Islam 201348 | 6m | -n: RA=92 | sc | per os (increasing dose) | – | -Analg | -Efficacy | 2c | |
| RCT | −74% ♀ | --> sc | -PIP | -Safety | ||||||
| -age 44 | ||||||||||
| -DD 4yr | ||||||||||
| 43 | Jundt 199349 | 4 wk | -n: RA=12 | sc/ im | per os | – | -PK | 3b-4 | ||
| O, P | –58% ♀ | per os--> im | ||||||||
| -age 58 | per os--> sc | |||||||||
| 44 | Katz 201550 | 6m | -n: RA=29 | sc | -Standard education | – | – | – | - Satisfaction | 3a |
| RCT | −62%♀ | -Video | ||||||||
| -age 49 | ||||||||||
| 45 | Lafforgue 199551 | 6m | -n: RA=46 | im | im | 11mg/ wk | -DMARDs | -Efficacy | 3a | |
| O, P | −63% ♀ | -Analg | -PK | |||||||
| -age 50 | -NSAID | -Safety | ||||||||
| -DD 6yr | ||||||||||
| 46 | Lambert 200452 | 22 wk | -n: RA=64 | im | per os--> im | 5mg/ wk | -Efficacy | 1b | ||
| RCT | −81% ♀ | -Safety | ||||||||
| -DD 9yr | ||||||||||
| 47 | Lee 201653 | 1 yr | -n: RA=35.640 | sc | per os | – | – | – | -Econ | 3b |
| O, P | per os--> biolog | |||||||||
| per os--> sc | ||||||||||
| per os--> sc-->biolog | ||||||||||
| 48 | Linde 200654 | > 2 yr | -n: RA=212 | im | per os--> im | 20mg/ wk | -Adhere | 3a | ||
| O, R | −71% ♀ | -Efficacy | ||||||||
| -age 51 | ||||||||||
| -DD 8yr | ||||||||||
| 49 | Luchikhina 201655 | 24m | -n: RA=191 | sc | sc (2 escalating rate) | -Efficacy | 3b | |||
| O, P | −82% ♀ | |||||||||
| 50 | Mainman 201056 | 6m | -n: RA=156 | sc | MTX sc vs. MTX per os | 5–30mg/ wk | -NSAID | -Efficacy | 3a | |
| C-C | −75% ♀ | -Cortic | -Econ | |||||||
| -age 54 | ||||||||||
| 51 | Michaels 198257 | < 2m | -n: RA=14 | iv | iv | -NSAID | -Efficacy | 4 | ||
| O, P | -Cortic | -Safety | ||||||||
| 52 | Michaud 201658 | 17 yr | -n: RA=22.621 | sc | MTX sc vs. MTX per os | -DMARDs | -Efficacy | 3 | ||
| O, R | −79% ♀ | |||||||||
| -age 59 | ||||||||||
| -DD 14yr | ||||||||||
| 53 | Moitra 200559 | – | -n=RA=102 | sc | per os --> sc | - Satisfaction | 3b-4 | |||
| O, R | ||||||||||
| 54 | Monjanel-Mouterde 199860 | – | -n: RA=34 | im | 10.5mg/ wk | -PK | 2c | |||
| CS | −67% ♀ | |||||||||
| -age 49 | ||||||||||
| 55 | Müller 201561 | 2 yr | -n: RA=70 | sc | sc | 20mg/ wk | -Efficacy | 2c | ||
| O, R | −57% ♀ | sc --> sc+biolog | -Safety | |||||||
| -age 58 | ||||||||||
| -DD 1.6yr | ||||||||||
| 56 | Müller-Ladner 201062 | 6m | -n: RA=128 | sc | scb --> sca | -NSAID | - Satisfaction | 2b | ||
| O, P | −73% ♀ | -Cortic | -Efficacy | |||||||
| -age 56 | -Safety | |||||||||
| -DD 3 yr | ||||||||||
| 57 | Myasoutova 201663 | 6m | -n: RA=43 | sc | MTX sc vs. MTX per os | 11.6mg/ wk | -DMARDs | -Efficacy | 3 | |
| RCT | −84% ♀ | -Safety | ||||||||
| 58 | Ng 200464 | >5yr | -n: RA=7,017 | sc | MTX scb vs. MTX per os | -Efficacy | 2a | |||
| O, R | ||||||||||
| 59 | O’Connor 201665 | 12m | -n: RA=103 | sc | sc | -Efficacy | 3 | |||
| O, R | −61. 4% ♀ | -Safety | ||||||||
| -age 56 | ||||||||||
| -DD 160 d | ||||||||||
| 60 | Oguey 199266 | 3 wk | -n: RA=10 | iv | iv--> per os dinner --> per os breakfast | -NSAID | -PK | 3a | ||
| O, P | −50% ♀ | -Cortic | ||||||||
| -age 58 | ||||||||||
| 61 | Osman 200167 | – | -n: RA=22; PM=1; IJA=1 | im | per os --> im | 17.5mg/ wk | – | -Efficacy | 4 | |
| CS | ||||||||||
| 62 | Pachon 201368 | 2 wk | -n: RA=104 | sc | – | – | – | - Satisfaction | 3b-4 | |
| 63 | Przygodzka 201769 | 3m | -n: RA=194 | sc | per os | – | – | – | -Efficacy | 3b |
| O, L | sc | -Safety | ||||||||
| 64 | Rau 199770 | 12m | -n: RA=174 | Im | im 7.5mg/ wk --> im 15mg/ wk | Not allowed | -NSAID | -Efficacy | 2b | |
| −60% ♀ | GS 25mg/ wk m --> GS 50mg/ wk | -Cortic | -Safety | |||||||
| -age 54 | ||||||||||
| -DD 11yr | ||||||||||
| 65 | Rau 200271 | 12m+2 yr | -n: RA=174 | im | im 7.5mg/ wk --> im 15mg/ wk | Not allowed 1st yr | -NSAID | -Efficacy | 2b | |
| RCT | −60% ♀ | GS 25mg/ wk m --> GS 50mg/ wk | -Cortic | |||||||
| -age 54 | ||||||||||
| -DD 11yr | ||||||||||
| 66 | Rawat 201672 | – | -n: RA=100 | sc | sc | – | – | – | Satisfaction | 4 |
| O, R | ||||||||||
| 67 | Rutkowska-Sak 200973 | – | -n: RA=70 | sc | per os--> sc | 15mg/ wk | – | – | -Safety | 4 |
| −91% ♀ | ||||||||||
| -age 55 | ||||||||||
| -DD 11yr | ||||||||||
| 68 | Sames 201474 | – | -n: RA=29 | sc | – | -- | – | – | Safety | 4 |
| -DD 15.3 | ||||||||||
| 69 | Sampaio-Barros 200075 | 1 yr | -n: EA=34 | im | 12.5mg/ wk | – | -NSAID | -Efficacy | 3a | |
| -Safety | ||||||||||
| 70 | Saraux 201976 | 6m | -n: RA=271 | sc | MTXa vs. self-injector | 15.4mg/ wk | -Efficacy | 2b | ||
| −75% ♀ | -Adhere | |||||||||
| -age: 59.2 | -Satisfaction | |||||||||
| -DD: 5.3 | -Safety | |||||||||
| 71 | Schiff 2014_277 | 8 wk | -n: RA=49 | sc | MTX per os vs. MTX sc (abd) vs. MTX sc (thigh) | – | -PK | 2a | ||
| RCT | −63.3% ♀ - | -Safety | ||||||||
| -age 61 | ||||||||||
| -DD 13yr | ||||||||||
| 72 | Schiff 201778 | 8 wk | -n: RA=49 | sc | per os--> sc | -PK | 2b | |||
| −63.3% ♀ | -Safety | |||||||||
| -age 61 | ||||||||||
| -DD 13yr | ||||||||||
| 73 | Scott 201479 | 5yr | -n: RA=196 | sc | per os--> sc | 17.7mg/ wk | – | -DMARDs | -Efficacy | 2c |
| −75.5% ♀ | -Safety | |||||||||
| -age 47 | ||||||||||
| 74 | Stamp 201180 | 6m | -n: RA=30 | sc | per os--> sc | 20mg/ wk | 5mg/ wk | -DMARDs | -Efficacy | 3a |
| O, P | −76.6% ♀ | -NSAID | -Safety | |||||||
| -age 51 | -Cortic | -Adhere | ||||||||
| -DD 7 yr | -PK | |||||||||
| 75 | Striesow 201281 | 5 wk | -n: RA=310; PsA=59 | sc | sca | – | -Satisfaction | 2b | ||
| O, P | −68.2% ♀ | -Safety | ||||||||
| 76 | Thompson 198482 | 12 wk | -n: RA=48 | im | PBO vs. MTX im | 15–25mg/ wk | – | -Efficacy | 1b | |
| −75% ♀ | -Safety | |||||||||
| -age 54 | ||||||||||
| -DD 13yr | ||||||||||
| 77 | Thornton 200883 | 6m | -n: RA=30 | sc | per os-->sc | 19.9mg/ wk | – | – | -Efficacy | 2c |
| −87% ♀ | -Safety | |||||||||
| -DD 15yr | ||||||||||
| 78 | Todoerti 201684 | 9yr | -n: RA=5,337 | sc | sc | -Safety | 3a | |||
| −68% ♀ | ||||||||||
| -age 63 | ||||||||||
| 79 | Wan 201785 | -n: RA=7,968 | sc | per os (--> increase dose) | -Efficacy | 2c | ||||
| per os--> sc | ||||||||||
| 80 | Wegrzyn 200486 | 6m | -n: RA=143 | im | im --> per os--> im | Yes | -Efficacy | 2c | ||
| −90% ♀ | -Safety | |||||||||
| -age 65 |
abd: abdomen; Adhere: adherence; analg: analgesics; RA: rheumatoid arthritis; inc: increasing; biolog: biologics; C-C: case-control; CPPD: calcium pyrophosphate deposition disease; CS: cross-sectional; CT: clinical trial; cortic: corticosteroids; cycles: cyclosporine; d/ wk: days per week; DD: duration of disease; DMARDs: disease-modifying anti-rheumatic drugs; Econ: economic; GS: gold salts; HCQ: hydroxychloroquine; IJA: idiopathic juvenile arthritis; im: intramuscular; m: months; max: maximum; mg/d: milligrams/ day; mg/ wk: milligrams/ weeks; MTX: methotrexate; NICE: National Institute for Health and Care Excellence; NSAID: non-steroidal anti-inflammatory drugs; O: observational; P: prospective; PBO: placebo; Ph-Ec: pharmaco-economic; PIP: proton inhibitor pump; PK: pharmacokinetics; PM: polymyositis; Ps: psoriasis; PsA: psoriatic arthritis; pt. edu: patient education; R: retrospective; RCT: randomized clinical trial; sc: subcutaneous; SLE: systemic lupus erythematosus; SpA: Spondylarthritis; ttm: treatment; WG: Wegener’s Granulomatosis; wk: week; yr: years.
Efficacy.
| Study / Dis | Indices of activity | APR | VAS / joint counts / HAQ | Survival | Others |
|---|---|---|---|---|---|
| Ahmed 2010 7 / RA | % ACR 20 (sc vs. oral): 57.5 vs. 9.1 | ||||
| % ACR 50 (sc vs. oral): 12.5 vs. 0 | |||||
| Bakker 20109 / RA | Response to ttm change (95% CI) | ||||
| -sc MTX=63 (50-70) | |||||
| -Cycles=48 (32–64) | |||||
| Branco 201610 / RA | Possibility of sc MTX withdrawal (95% CI) | ||||
| −1 yr=6.1% (0–9.5) | |||||
| −2 yr=8.5% (0.1–16.1) | |||||
| −3 yr=23.2% (5.3–37.7) | |||||
| Bharadwaj 200711 / RA | -DAS28 final <5.1=87.5% | ||||
| -DAS28 final <3.2=37.5% | |||||
| -Δ DAS 28>1.2=71.8% | |||||
| Bianchi 201812 / RA | -DAS28-GSR remission=44.4% | ||||
| -ΔDAS28>1.2=88.8% | |||||
| Bingham 200313 / RA | -Δ DAS28 s12=5.8 (P=.015) | -↓CRP s12 (NS) | -s12=88% | Corticosteroid dose | |
| -Δ DAS28 s24=5.7 (P=.014) | -↓CRP s24 (P=.022) | -s24=58% | -s12: No change | ||
| -s24: ↓ prednisone (NS) | |||||
| Borman 201414 / RA | 1 m | 1 m | 1 m | ||
| -DAS 28=3.6 (P<.010) | -GSR:33.8 (P<.050) | - VAS pain: 53.9 (P<.050) | |||
| 3m | -CRP:1.4 (P<.050) | 3m | |||
| -DAS 28=3.4 (P<.010) | 3m | - VAS pain: 51.6 (P<.050) | |||
| -GSR:29.7 (P<.050) | |||||
| -CRP:0.8 (P<.050) | |||||
| Braun 200815 | 6m (sc vs. oral) | sc MTX vs. oral MTX | s16 | ||
| -ACR20: 78 vs. 70 (P<.050) | -NSJ: 2 vs. 3 (P=.040) | n=52 (14%) non-responders | |||
| -ACR 50: 62 vs. 59 (NS) | -NPJ: 3.5 vs. 6 (NS) | -Change oral MTX -- > sc MTX (n=30, 30% ACR20 6m) | |||
| -ACR 70: 41 vs. 33 (P<.050) | -HAQ: 0.4 vs. 0.5 (NS) | -Change sc MTX15mg -- > 20mg (n=22. 23% ACR20 6m) | |||
| -DAS28: 3.3 vs. 3.7 (P not shown) | |||||
| Burbage 200117 / RA, SpA, SLE | Improvement GSR and CRP at 3 and 9m (P<.01 both) | ||||
| Chichasova 201821 / RA | LDA | -Minimal radiographic progression: 24% | |||
| --3m=18 ptt | |||||
| --6m=51% | |||||
| --12m=81% | |||||
| --36m=64% | |||||
| Remission | |||||
| --12m=19% | |||||
| --36m=36% | |||||
| De Groot_199724 / GW | −7 ptt with response | ||||
| --6 complete response | |||||
| --4 partial remission | |||||
| -Mean dose of prednisone=1.75mg / d | |||||
| --7 ptt with complete withdrawal of prednisone | |||||
| Dhaon 201326 / RA | -SDAI | ||||
| --Fractionated vs injected dose (P=.005) | |||||
| Dhaon 201727 / RA | MTX vs / 2s vs. oral MTX vs. im MTX | ||||
| -LDA (%)=49 vs. 35.5 vs. 47 | |||||
| ΔSDAI=−8 (±4.5) vs. −0.1 (±7.6) vs. −6 (±7.2) | |||||
| Finckh 201428 / CPPD | MTX vs. PBO | MTX vs. PBO | MTX vs. PBO | MTX vs. PBO | MTX vs. PBO |
| DAS44=−0.08 vs. −0.13 | CRP=.2 vs. 0.3 | NPJ=0 vs. −1 | Withdrawals 5 vs. 0 | No. analgesics=0 vs. 0 | |
| NSJ=−1 vs. 0 | No. flare ups / 3m=0 vs. 0 | ||||
| VAS pain=−1 vs. 0 | |||||
| Gottheil 201634 / RA | sc MTX vs. MTX oral and need for biologics-- > HR=0.47; P=.015 | ||||
| Gridneva 201636 / RA | As per BMI≤25 vs. 25–30 vs.≥30 | As per BMI≤25 vs. 25–30 vs.≥30 | |||
| 3m | Mean dose of sc MTX (mg / wk) = | ||||
| -Remission ACR / EULAR 2011=20 vs. 6 vs. 0 | --3m=12.7 vs. 11.3 vs. 10.3 | ||||
| 6m | --6m=13.3 vs. 11.5 vs. 9.4 | ||||
| -DAS 28=2.1 vs. 2.6 vs. 30=3 | --12m=13.1 vs. 11.5 vs. 10.2 | ||||
| -SDAI=4.3 vs. 5.5 vs. 10.0 | Need for use of DMARD (%)=23 vs. 60 vs. 60 | ||||
| -Remission ACR / EULAR 201=30 vs. 24 vs. 10 | |||||
| 12m | |||||
| -DAS 28-GSR=2.0 vs. 2.9 vs. 2.4 | |||||
| -DAS 28-CRP=1.7 vs. 2.4 vs. 2.3 | |||||
| -SDAI=1.2 vs. 3.3 vs. 4.3 | |||||
| -Remission ACR / EULAR 2011=60 vs. 30 vs. 30 | |||||
| Griffin 200438 / RA | -↓ NSJ (P<.05) | -At 6m | |||
| -↓ NPJ (P<.01) | -↓ use cortic (P<.03) | ||||
| -↓ pain (P<.01) | -↑ haemoglobin (P<.05) | ||||
| -↓ PGA (P<.02) | |||||
| -↓ PhGA (P<.02) | |||||
| -↓ HAQ (NS) | |||||
| Haibel 200739 / AS | -ASAS20=25% | -CRP=1mg / dl -- > 0.8mg / dl | -Medical spine pain=no improvement | −4 withdrawals inefficacy (2 in wk 4 and 2 in wk 12) | |
| -ASAS40=10% | -NSJ=4.7 -- > 1.2 | ||||
| -ASAS70=0 | -N° entesis=2.2 -- > 1.9 | ||||
| -ASAS partial remission=0 | |||||
| -BASDAI20=30% | |||||
| -BASDAI50%=10% | |||||
| -BASDAI70=5% | |||||
| -ΔBASDAI=0 | |||||
| Hameed 201040 / RA | -Δ DAS28 ineff group 3month 4.2 (P=.006) | ||||
| -Remission ineff group n=4 (10%) | |||||
| -Δ DAS28 group 3-month AE 3 43 (P<.001) | |||||
| -Remission AE group n=21 (33%), 6 already in remission at the start | |||||
| Hammond 2015 42 / RA | -Inadequate response to oral MTX (tolerability) (n=20) | -Mean duration of sc MTX in monotherapy | |||
| -DAS 28 pre-change vs. post-change: 4.46 vs, 3.65 | -Inadequate response to oral MTX (tolerability)=28.6m | ||||
| -DAS28≤3.2=40% | -Inadequate response to MTX vol (efficacy)=7 m | ||||
| -DAS28≤2.6=30% | |||||
| -Inadequate response to oral MTX (efficacy) (n=29) | |||||
| -DAS28 pre-change vs. post-change: 5.34 vs. 4.08 | |||||
| -DAS28≤3.2=24% | |||||
| -DAS28≤2.6=7% | |||||
| Harris 201843 / RA | HR (probability of ttm change): 0.64 (95% CI 0.52–0.78) | ||||
| Hazlewood 2016 45 / RA | (Estimated data for sc MTX) | -HAQ: HR=−0.02 (−0.13, 0.10); P=.75 | |||
| -Difference DAS 28 between ttm groups: HR=−0.38 (−0.64, −0.10); P=.04 | |||||
| -Remission DAS 28: OR=1.02 (0.96, 1.06); P=.002 | |||||
| -Sustained remission DAS 28: OR=1.02 (0.96, 1.06); P=.43 | |||||
| Huber 200647 / Cutaneous LE | -Improvement of lesions | ||||
| Islam 201348 / RA | -sc MTX vs. oral MTX | -sc MTX vs. oral MTX | -sc MTX vs. oral MTX | ||
| -ACR20 (%)=93 vs. 80 (P=.020) | -GSR=43 vs. 50 (P=.03) | -NPJ=21 vs. 31 (P=.020) | |||
| -ACR50 (%)=89 vs. 72 (P=.030) | -NSJ=4 vs. 8 (P=.030) | ||||
| -ACR70 (%)=11 vs. 9 (NS) | -Pain=2 vs. 3 (NS) | ||||
| -PGA=2 vs. 3 (NS) | |||||
| -PhGA=2 vs. 3 (P=.020) | |||||
| -HAQ=7 vs. 9 (P=.04) | |||||
| -MS (min)=25 vs. 38 (NS) | |||||
| Lafforgue 199551 / RA | Responders (n=32, 70%) vs. non-responders (n=14, 30%) | ||||
| Lambert 200452 / RA | -im MTX increasing doses vs. im MTX control | -Δ GSR: 2, vs. −5.4 (NS) | -Δ NSJ: −1 vs. −2 (NS) | -Patient with infiltrations of cortic 59% vs. 37% | |
| -DAS28<3.2=3.7 vs. 3.7 (NS) | -Δ NSJ: −4 vs. −3 (NS) | -No. infiltrations of cortic 20 vs. 12 | |||
| -Δ DAS28>1.2=18.5 vs. 18.5 (NS) | -Δ VAS PGA: −12 vs. −10 (NS) | ||||
| -ACR20=3.7 vs. 3.7 (NS) | -Δ VAS PhGA: −3.5 vs. −3.6 | ||||
| -EULAR response good=0 vs. 0 (NS) | -Δ VAS pain: 9 vs. −18 | ||||
| -EULAR response moderate=30 vs. 36 (NS) | - Δ HAQ: 0.05 vs. 0.14 (NS) | ||||
| -No EULAR response=70 vs. 74 (NS) | |||||
| -SF-12: NS (data not shown) | |||||
| -Δ DAS28: −0.5 vs. −0.7 (NS) | |||||
| Linde 200654 / RA | -Δ CRP 6m: 20 to 12 (P>.001) | -Use of cortic at m: 66% to 46% (P>.001) | |||
| Luchikhina 201655 / RA | 12m | % ptt with change to biologic=63.9 | |||
| -% SDAI low activity=38.2 | At 12m (rapid escalation vs. slow escalation) | ||||
| -% SDAI remission=34 | -% in monotherapy: 49.4 vs. 25 | ||||
| -% change to biologic: 50.6 vs. 75 | |||||
| At 24m (rapid escalation vs. slow escalation) | |||||
| -% in monotherapy: 46.3 vs. 19.7 | |||||
| -% change to biologic: 53.7 vs. 80.3 | |||||
| Mainman 201056 / RA | sc MTX vs. oral MTX | -Δ GSR=N) | -Δ Pain=NS | ||
| -% DAS28>1.2=74 vs. 48 (P=.035) | |||||
| -% DAS28>3.2=92 vs. 16 (P=.002) | |||||
| - %EULAR response≥good (sc MTX): 58 | |||||
| Michaels 198257 / RA | -Δ GSR: 63 to 38 | -Δ MS: 6 to 2.5 | |||
| -Δ joint count: 57 to 33 | |||||
| Michaud 201658 / RA | Median survival: sc MTX vs oral MTX=1.5 (0.5–3.5) vs. 2 (1–5.5) | ||||
| -% change between both admon=16.2 | |||||
| Müller 201561 / RA | 6m/12m / 18m/24m | 6m/12m / 18m/24m | -Withdrawal rate 45.7% (sc MTX vs. sc MTX+biologic 46% vs. 45% (NS) | -Mean doses sc MTX vs. sc MTX+biologic 17.4 vs. 19.1 (NS) | |
| -All ptt | -All patients | ||||
| -DAS28: 2.70 / 2.45 / 2.50 / 2.51 | -GSR:9 / 9 / 8 / 8 | ||||
| -% DAS28<3.2: 80 | -CRP:3.23 / 3.20 / 3.78 / 3.85 | ||||
| -%DAS28<2,): 72.9 | -sc MTX monotherapy | ||||
| -sc MTX monotherapy | - GSR: 7 / 7 / 7 / 7 | ||||
| -DAS28: 2.11 / 1.92 / 1.93 / 1.84 | -CRP: 2.66 / 2.53 / 3.22 / 3.12 | ||||
| -%DAS28<3.2: 81.1 | -sc MTX+biologic | ||||
| -%DAS28<2.6: 75.7 | -GSR: 10 / 8 / 9 / 9 | ||||
| -sc MTX+biologic | -CRP: 3.61 / 3.52 / 4.07 / 4.2 | ||||
| -DAS28: 3.18 / 2.81 / 2.81 / 2.89 | |||||
| -%DAS28<3.2: 78.8 | |||||
| -%DAS28<2.6: 69.7 | |||||
| Müller-Ladner 201062 / RA | 20ml vs. 50ml | ||||
| -VAS-PGA=63.5 vs. 95 (P<.001) | |||||
| -VAS PhGA=82 vs. 96 (P<.001) | |||||
| Myasoutova 201663 / RA | sc MTX vs. oral MTX | ||||
| - ACR 20 (ptt)=23 vs. 14 | |||||
| - DAS 28 at 6m=2.3 vs. 1.3 (P<.05) | |||||
| Ng 200464 / RA | -Probability of change with sc MTX HR=0.64 (95% CI 0.52–0.78) | ||||
| O’Connor 2016 65 / RA | Baseline / 6s / 12s | ||||
| CDAI | |||||
| - ≤ 2.8=0 / 16.7 / 19.1 | |||||
| −2.9-10.0=8.8 / 26.0 / 53.9 | |||||
| −10.1- 22.0l=25.5 / 38.5 / 23.6 | |||||
| - > 22=65.7 / 18.8 / 3.4 | |||||
| SDAI | |||||
| - ≤ 3.3=0 / 6.9 / 8.1 | |||||
| −3.4-11.0=3.1 / 22.4 / 43.2 | |||||
| −10.1- 22.0=15.5 / 32.8 / 35.1 | |||||
| - > 26=81.4 / 37.9 / 13.5 | |||||
| DAS 28 | |||||
| - ≤ 2.4=2.1 / 25.9 / 30.3 | |||||
| −2.5-3.6=9.6 / 32.9 / 43.4 | |||||
| −3.7- 5.5=42.6 / 34.1 / 25.0 | |||||
| - > 5.5=45.7 / 7.1 / 1.3 | |||||
| Osman 200167 / RA | CRP=53 -- > 34mg / l | −20 ptt improved | |||
| −3 got worse | |||||
| −1 no change | |||||
| Przygodzka 201769 / RA | 3m | ||||
| oral-- > sc=26% | |||||
| sc-- > oral=4% | |||||
| Cortic=12% of oral group | |||||
| Rau 199770 / RA | -im MTX Δ measured at 0m/6m / 12m | -im MTX Δ measured at 0m/6m / 12m | -im MTX Δ measured at 0m/6m / 12m | -Marked improvement 68% vs. 76% (NS) | |
| -Lansbury index: 64.6 / 31.3 / 29 (P<.05) | -GSR: 41.5 / 22.8 / 21.1 (P<.05) | -NPJ: 18.4 / 11.9 / 11.4 (P<.05) | -Ttm failure 14% vs. 19% (NS) | ||
| -Clinical remission MTX vs. GS 11.5% vs. 24.1% P<.050 | -CRP: 4.1 / 2.2 / 2.5 (P<.05) | -NSJ: 14.9 / 8 / 7.6 (P<.05) | |||
| -MS: 3.4 / 2.2 / 2.1 (P<.05) | |||||
| -Art pain: 3.6 / 2.7 / 2.8 (P<.05) | |||||
| -Str right hand: 0.3 / 0.5 / 0.5 (P<.05) | |||||
| -ADL: 69.1 / 81.1 / 78.4 (P<.05) | |||||
| Rau 200271 / RA | im MTX-- > 12m/24m / 36m | ||||
| -Ratingen score=11.9 / 14 / 17.6, P<.05 vs. baseline, NS vs. GS (at any timepoint) | |||||
| -Articulaciones with erosiones=8.7 / 10.2 / 11.1 P<.05 vs. baseline, NS vs. GS (at any timepoint) | |||||
| -Ratingen score ≤ 5%=60 / 54.1 / 50 NS vs. GS (at any timepoint) | |||||
| -Ratingen score 6%–10%=22.7 / 25.7 / 22.9 NS vs. GS (at any timepoint) | |||||
| -Ratingen score 11%–20%=13.3 / 13.5 / 15.7 NS vs. GS (at any timepoint) | |||||
| -Ratingen score >20%=4 / 6.8 / 11.4 NS vs. GS (at any timepoint) | |||||
| -Progression < 2nd than 1st year (Retinger score and erosions) P<.050 | |||||
| Sampaio-Barros 200075 / AS | -↓ GSR (P<.001) | −31 patients completed ttm | −53% considered responders | ||
| Saraux 201976 / RA | % change HAQ (Self-injector vs. pre-filled pen)=20.4 vs. 20.3 | ||||
| Scott 201479 / RA | -% Survival of sc MTX | ||||
| −1 yr=83 | |||||
| −2 yr=75.2 | |||||
| −5yr=47 | |||||
| Stamp 201180 / RA | -Δ DAS28 (median) 0–6m | -Δ median 0–6m | -Median change | ||
| - 3.27 vs. 2.56 (NS) | -GSR: NS | -NSJ: 2 vs. 0 (P=.001) | |||
| -CRP: NS | -NPJ: NS | ||||
| -HAQ modified: 0.5 vs. 0.125 (P=.030) | |||||
| -Pain: 24.5 vs. 17 (P=.014) | |||||
| -PGA: 29.5 vs. 16 (P=.004) | |||||
| -Fatigue: NS | |||||
| Thompson 198482 / RA | -Δ GSR (6 wk) im MTX vs. PBO=29 vs. 43 (P<.001) | -Mean Δ (6 wk) im MTX (10 and 25mg / wk) vs. PBO | |||
| Ptt with Important clinical improvement | -NSJ: 18 vs. 35 (P<.001) | ||||
| --im MTX 10mg / wk vs. PBO=6 vs. 0 (P<.010) | -NPJ: 25 vs. 55 (P<.002) | ||||
| --im MTX 25mg / wk vs. PBO=6 vs. 0 (P<.005) | -PhGA: 69 vs. 38 (P<.001) | ||||
| -Pain: 33 vs. 65 (P<.001) | |||||
| -MS: 0.9 vs. 3.8 (P<.005) | |||||
| -Hand str=126 vs. 97 (P<.005) | |||||
| -t to walk 50 steps=12.6 vs. 14.2 (NS) | |||||
| - Ptt with Important clinical improvement | |||||
| im MTX 10mg / wk vs. PBO | |||||
| NSJ: 5 vs. 0 (P<.050) | |||||
| -NPJ: 6 vs. 2 (NS)- | |||||
| -PhGA: 10 vs. 2 (P<.01) | |||||
| -Pain: 8 vs.1 (P>.010) | |||||
| -MS: 10 vs. 2 (P<.01) | |||||
| -Fatigue: 4 vs. 3 (NS) | |||||
| -Hand str 1 vs. 0 (NS) | |||||
| -t to walk 50 steps: 0 vs. 0 (NS) | |||||
| im MTX 25mg / wk vs. PBO | |||||
| -NSJ: 1 vs. 0 (NS) | |||||
| -NPJ: 6 vs. 2 (P<.050) | |||||
| -PhGA:6 vs. 2 (P<.010) | |||||
| -Pain: 5 vs. 1 (P<.050) | |||||
| -MS: 7 vs. 2 (P<.010) | |||||
| -Fatigue: 6 vs. 3 (NS) | |||||
| -Hand str: 2 vs. 0 (NS) | |||||
| -t to walk: 0 vs. 0 (NS) | |||||
| Thornton 200883 / RA | At 3 / 6m | At 3 / 6m | |||
| -↓ median DAS28: 2.34 (P<.001) / 2.09 (P<.001) | -Use of anti-TNFα: 0 / n=3 ptt | ||||
| -Good EULAR response: 74% / 52% | |||||
| Wan 201785 / RA | Reason for probability of initiating biologic ttm (reference category increase oral dose): 1.06 (95% CI 0.82–1.38; P=.635) | ||||
| Wegrzyn 200486 / RA | (↑ / ↓ / no change) | -im MTX -- > oral 3m | |||
| 3m | Analgesic use 66% / 0% / 31% (P<.001) | ||||
| -im MTX -- >oral | -Duration analgesic use 66% / 0% / 31% (P<.001) | ||||
| -Morning pain 49 / 0 / 41 (P<.001) | -Dry eye 14% / 0% / 57% (NS) | ||||
| -MS: 64 / 0 / 34 (P<.001) | -Dry. mouth 19% / 0% / 50% (NS) | ||||
| -t art stiffness: 63 / 0 / 34 (P<.001) | -oral MTX -- > im ↑ with oral followed by ↓ following im | ||||
| -Art pain: 71 / 0 / 29 (P<.001) | -Analgesic use 63% (P<.001) | ||||
| -Art. inflammation: 59 / 0 / 34 (P<.001) | -Duration analgesic use 65% (P<.001) | ||||
| -oral MTX -- > im ↑ with oral followed by ↓ after im | -Dry eye, no change 47% | ||||
| -Morning pain=42% (P<.001) | -Dry mouth, no change 40% | ||||
| -MS: 49% (P<.001) | |||||
| -t art stiffness: 60% (P<.001) | |||||
| -Art pain: 70% (P<.001) | |||||
| -Art. inflammation: 40% (P<.001) |
ACR: American College of Rheumatology; admon: administration; AE: adverse events; ADL: activities of daily living; APR: acute phase reactant; art: articular; AS: ankylosing spondylitis; ASAS: Assessment of SpondyloArthritis International Society; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BMI: body mass index; CDAI: Clinical Disease Activity Index; CI: confidence interval; cortic: corticosteroids; cycles: cyclosporine; CRP: C reactive protein; DAS: Disease Activity Score; Dis: disease; DMARD: disease-modifying anti-rheumatic drug; GS: gold salts; HAQ: Health Assessment Questionnaire; im: intramuscular; ineff: inefficacy; LDA: low disease activity; m: months; MS: morning stiffness; MTX: methotrexate; NPJ: number of painful joints; NS: not significant; NSJ: number of swollen joints; PBO: placebo; PGA: Patient global assessment; PhGA: Physicians’ global assessment; ptt: patient; RA: rheumatoid arthritis; sc: subcutaneous; SDAI: Simplified Disease Activity Index); SLE: systemic lupus erythematosus; SpA: Spondylarthritis; str: strength; TNF: tumour necrosis factor; t: time; ttm: treatment; oral: route oral; GSR: glomerular sedimentation rate; VAS: visual analogue scale; wk: weeks; yr: years.
Despite the fact that most of the studies included examined the efficacy of parenteral MTX, most of them did not have a comparator group. Moreover, as older studies were included, currently used efficacy endpoints, such as remission or EULAR response, could only be found in the most recent studies. The domains assessed include data on activity (including remission), structural damage, pain, stiffness, function, fatigue, hand strength, physician and patient global ratings, quality of life, acute phase reactants, corticosteroid consumption and, as a possible efficacy variable, saving/ delayed prescription of biologic therapies, MTX survival, and treatment changes.
Rheumatoid arthritisThe most robust evidence would come from randomised clinical trials (RCTs) with a parallel comparator group. In the RCT by Braun et al.,15 comparing the efficacy of oral MTX with sc MTX at the same doses over a 6-month period in MTX-naïve RA, mostly at baseline, they found statistically significant superiority of the sc formulation in the composite response rates ACR20 and 70, with no difference in ACR50, although the percentage was higher with the subcutaneous formulation, and in the reduction in the number of swollen joints, but not in the number of painful joints. Nor were differences found in HAQ and, as for the DAS28 activity index, the 6-month figure for sc MTX group 3.3 vs. 3.7 in oral MTX (P-value not shown). On the other hand, in the subgroup of patients who failed with the oral formulation and switched to sc at week 16, 30% achieved ACR20 at 6 months.
Continuing with RCTs, in the 6-month study by Islam et al.,48 patients refractory to oral MTX were randomised to either oral dose escalation or sc MTX. When comparing both formulations, again for many of the variables, the sc formulation was significantly superior to the oral formulation, specifically for ACR20 and 50, but not 70. Despite a higher percentage for the sc formulation (11% vs. 9%), the number of painful joints (NAD), physician global assessment (GMV), HAQ, and erythrocyte sedimentation rate (ESR), there was no difference in pain, patient global assessment (PGV), and morning stiffness.
In other RCTs and in the observational studies included, the comparison between MTX formulations is sequential and not in parallel groups. Here we found that, in patients who switched oral MTX because of inefficacy and toxicity, therapeutic response can be achieved, with different magnitudes of effect. Most of the follow-ups are not very long (several months; occasionally data are available for up to 3 years), although data are beginning to be reported indicating that the efficacy of the drug can be maintained over longer periods of term.42
Notably, a subgroup analysis in the study conducted by Gridneva and Muraviev in 201636 compared the effect of MTX via the sc route in terms of body mass index (≤25kg/m2 vs. >), with a higher percentage of improvement in individuals having a lower body mass index.
Spondyloarthritis (SpA)Only one study in SpA probed the efficacy of sc MTX, and only indirectly at that. The study by Burbage et al. in 200117 included data regarding several rheumatic diseases, including four patients with SpA. Efficacy was assessed using acute phase reactants. This revealed that in patients with poor voxel tolerance, switching to the parenteral route was associated with improvement in these parameters.
Other diseasesAlmost anecdotally, studies were found involving other diseases. In one study with two patients in systemic lupus erythematosus (SLE)17 and another with a single patient with polymyositis,67 effectiveness was examined by analysing the decrease in CRP values, which was achieved in both diseases. Both studies are also of low quality. Likewise, we found one study that probed the efficacy of sc MTX for the treatment of calcium pyrophosphate deposition disease (n=26),28 with no clear result in favour of the treatment group versus placebo.
SafetyAlmost two thirds of the studies included in this review looked at the safety and tolerability of parenteral MTX, some comparing it with orally administered MTX, others with different doses of parenteral MTX, but most did not have a comparator group (appendix 2). As with efficacy, the comparison between oral and parenteral formulation has not been done with parallel designs, but sequentially. Regardless of the pharmacokinetics of MTX, this cannot entirely avoid the existence of residual effects that bias the results obtained with the injected route. It should be noted that the type of adverse event (AE) was not always well defined, nor are the heterogenous ways of grading their severity, and there is tremendous variability in how they are defined and recorded.
In general, in both RCTs,15,48 and observational studies (regardless of how treatments were compared), parenteral MTX does not increase the rate, type, or severity of AEs described for oral MTX. Nor have changes been observed with different doses of parenteral MTX or when comparing different concentrations of the sc formulation.
It has been suggested that injected administration of MTX could possibly have a favourable effect on the rate of gastrointestinal AEs relative to the oral formulation. One of the included RCTs notes this,48 but not the RCT by Braun et al.15 In fact, the authors commented in the Discussion on this finding as unexpected. There is one observational study (of low quality) that specifically compared the intensity of gastrointestinal AEs between doses of 7.5 and 15mg/week in the two formulations. In general (although it depends on the type of AE), the intensity was higher with the oral formulation.
Similarly, it has been suggested that the injected route may improve the findings on transaminase levels reported with the oral route. The RCT by Braun et al.15 found elevated transaminases in 4.3% of patients on MTX per os and in 1.6% on the parenteral formulation. Comparative studies are lacking to definitively confirm this point.
AdherenceFour observational studies of moderate-good quality and one CE of low level of evidence stand out, in which adherence to parenteral treatment was directly assessed (albeit using different definitions). However, they lack a comparator group, and in the first one described, the inclusion criterion was that the subject was a “potential adherent” (Appendix 3). It should be noted that these are all studies with few patients, so the validity of the results is very limited.
The first of these studies (apparently short-term, although of unclear duration) examined self-reported adherence to parenteral drugs.8 Forty patients with RA and psoriatic arthritis (PsA) were included, 20 of whom initiated im MTX. These participants were provided with education and training for self-injection, and reported 92.5% adherence, defined as ≥80% compliance with the prescribed schedule.
Another study54 found that in 12 patients with rheumatic disease who switched from MTX per os to im at 6months, there was no difference in adherence to MTX im, given the discontinuation of oral MTX due to inefficacy or AE. Stamp et al.,80 after analysing 30 RA patients who switched from MTX per os to sc MTX, found only one non-adherent patient at 6 months.
The study by Flipo et al.31 (low quality), which measured compliance using the Morisky index, revealed moderate adherence. In this work, physicians considered their patients’ adherence to be higher than the patient-reported compliance.
A few studies evaluated the persistence of parenteral MTX,10,54 one of which was only found in abstract form at congress.42 Drug persistence has been seen to decrease over time and that injected MTX persists longer than MTX per os.
It is important to take into account the favourable role that education and training for parenteral administration, the use of prefilled syringes, and the smaller volume of syringes may have on adherence.8,62,81
SatisfactionStudies assessing satisfaction are highly heterogeneous in both form and content and of medium-low quality (Annex 4). In general, all the studies report good or very good satisfaction, above 70%, although in each of them, the analysis of satisfaction was approached from a different perspective; for example, satisfaction with the change of drug delivery route,59 the type of device used for parenteral administration,25,81 or even pain at the site of injection,32 among others. Given the characteristics of the design and quality [of the studies], it is difficult to draw definitive conclusions in this category.
Pharmacokinetic studiesThe results found with respect to pharmacokinetics are shown in Annex 5. The evidence on differences in bioavailability between oral and parenteral formulations is based on one 8-week, good quality, crossover RCT, as well as multiple small-sample, short duration, moderate quality, observational studies.41,46,49,51,66 The pharmacokinetics of both sc, im, and injected MTX at different doses have been evaluated.
With MTX per os, it has been observed that as the dose increases, its bioavailability decreases, reaching a plateau at 15mg/week, a phenomenon that is not observed with parenteral MTX. In other words, from 15mg/week onwards, the bioavailability of parenteral MTX would be higher than that of the oral route, and is similar at lower doses. Also based on these findings, one of the studies46 suggested that to achieve greater efficacy in individuals with MTX25mg/week, they should switch to the parenteral route.
Schiff and Sadowski78 suggest a formula based on pharmacokinetics for dose escalation from oral to parenteral (y=0.6101x+2.9274), which could have clinical applicability.
Pharmaco-economic analysisThe systematic review included a quality study on the cost-effectiveness of parenteral MTX in RA patients naïve to MTX, adjusted to the peculiarities (including costs) of our national health system.22
Other studies have performed other drug-economic analyses, specifically cost minimisation.29,30 Using UK costs, the use of sc MTX in patients refractory to oral MTX can save £7,197 per patient in the first year and £9.3million per year in new patients. Other studies56 have estimated that, for every 1,000 RA patients, 40 are being treated with sc MTX, and, taking into account the fact that 36 of these patients have a response equivalent to that of an anti-TNFα, the savings (from using sc MTX instead of biologic therapy) are estimated to be £306,000, or £300 per patient-year. All pharmacokinetic studies found can be found in Annex 6. The difference in health systems and the difference in the variables used in each study, as well as their quality, make it difficult to draw adequate conclusions.
DiscussionAlthough MTX is currently the cornerstone of initial treatment in RA, it is often not sufficiently well tolerated by patients and the possibility is always raised of changing the route of administration to achieve better adherence and fewer AEs. The aim of this review is to study the phenomenon from the clinical perspective of efficacy, adherence, and safety, as well as from the perspective of economics and patient satisfaction, aspects that should not be underestimated in clinical practice.
In terms of efficacy, it is of interest to explore the switch from per os to sc in those individuals who may not be achieving optimal results in terms of controlling their RA and to do so without increasing AEs. It would therefore appear that efficacy in RA correlates more with the dose than to the route of administration, although the same cannot be concluded in EspA or in other systemic autoimmune diseases, due to the scarce data found. Switching to the parenteral route may increase efficacy in some cases; it would therefore be interesting to carry out studies on specific profiles. In general, with MTX, when a clinical response is insufficient, a dose increase can be assessed, due to the almost linear relationship between dose and observed effect.87,88
Despite the low quality of the studies to assess the safety of parenteral MTX - in many of them there is no comparator group or the comparison with oral MTX is not conducted in parallel, so that a residual effect of the latter may be observed - there is a certain tendency for side effects to be more intense with oral MTX, including a greater elevation of transaminases.15,66
With regard to the evaluation of adherence, there is also the problem of the quality of the studies and the different parameters used to assess compliance. In general, adherence is good in the parenteral route, a fact that may be strongly influenced by the need for education regarding the devices for its administration. In this regard, nurses play a key role in the early detection of side effects, comorbidities, and in education on issues related to their disease, including the need for adherence.89
In general, patients are satisfied with parenteral use, although most studies in this area evaluate different pharmacological presentations,25,32,81 making it difficult to assess satisfaction with the route per se. Indirectly, good adherence and persistence of the drug can be taken to indicate that it is due to patient satisfaction with its use. It is worth bearing in mind that as parenteral MTX is used for generally higher doses (and hence, in patients with more severe disease), there may be greater awareness of need, a critical aspect for adherence.4
Most studies that probe a second line in RA favour biologics and sc MTX is not included in the scenarios. However, switching from oral to parenteral administration in individuals who have failed to achieve an adequate response is also advantageous for efficacy reasons, as it may avoid switching to biologics.27,15,90 The drug-economic analysis of parenteral MTX reveals savings by achieving suitable optimisation of treatment and avoids the use of biologics inasmuch as possible, with their consequent economic burden on the system and hardship of side effects, particularly infections.
Despite trying to cover as many aspects of interest surrounding MTX as possible, it is difficult to draw precise conclusions. This may be because the level of evidence in the papers found is medium-low and, in many cases, the scant sample size also limits the extrapolation of conclusions. Similarly, the lack of a common outcome variable in the different settings analysed also comprises another limitation. Based on the results of this review, further studies of the use of injected MTX in other systemic autoimmune diseases are needed, as the results obtained do not allow for valid conclusions.
With the inherent limitations given the available evidence, parenteral MTX could be an alternative to the use of oral MTX if there is a need for dose increase and there are gastrointestinal problems, due to its efficacy, safety, adherence and pharmaco-economic results, especially in patients with RA.
Conflict of interestThe authors have no conflict of interests to declare.
Please cite this article as: Otón T, Carmona L, Loza E, Rosario MP, Andreu JL. Revisión sistemática del uso de metotrexato por vía parenteral en enfermedades reumáticas. Reumatol Clin. 2022;18:207–226.