Enthesitis is one of the characteristic etiopathogenic manifestations of spondyloarthritis. However, in clinical practice, its presence often goes unnoticed because of the lack of precision and sensitivity of physical examination to detect it. Viable, valid and reliable imaging tests are needed for early diagnosis, as well as a good sensitivity to change to monitor therapeutic response. In this paper we review the most relevant aspects of current knowledge of the enthesis and discuss the validity of ultrasound for assessing enthesitis in spondyloarthritis and its sensitivity to change to monitor therapeutic response.
La inflamación de la entesis es una de las manifestaciones etiopatogénicas características de las espondiloartritis. Sin embargo, en la práctica clínica, su presencia pasa muchas veces desapercibida debido a la falta de precisión y de sensibilidad de la exploración física para detectarla. Son necesarias pruebas de imagen viables, válidas y fiables para un diagnóstico precoz, y con buena sensibilidad al cambio para monitorizar respuesta terapéutica. En este trabajo, se revisan los aspectos más relevantes de los conocimientos actuales de la entesis y se analiza la validez de la ecografía para valorar entesitis en pacientes con espondiloartritis, así como su sensibilidad al cambio para monitorizar respuesta terapéutica.
The enthesis is considered the target tissue for inflammation in spondyloarthritis (SpA) and is key in the pathogenesis of this group of diseases. From a comparative point of view, we can say that the enthesis is for SpA what the synovium is in rheumatoid arthritis (RA). RA is characterized by both arthritis and tenosynovitis, with enthesis involvement being non-existent or very minor. However, in SpA inflammation occurs both in entheses and in the synovial tissue. McGonagle has shown that synovitis that occurs in patients with SpA is secondary to the release of proinflammatory cytokines from the entheses, unlike RA, where a primary autoimmune synovitis1 is produced. This hypothesis is based on studies of magnetic resonance imaging (MRI) of the knee comparing patients with RA and SpA; patients with SpA had enthesitis and synovitis in the same joint, while patients with RA only had synovitis.2
The concept of SpA as a group appears in 1958, advocated by the theory of “separatists”, who begin to see it as its own entity, on the basis of common clinical features in the patients with this condition which clearly differed from RA.3 However, it was not until 10 years later, in 1970, when the involvement of entheses was first described in the pathogenesis of SpA.4 Subsequently, it has been demonstrated that inflammation of the enthesis is responsible for many of the symptoms, and explains the multitude of locations of pain in these patients. Thus, at an axial level, it is responsible for inflammatory back pain, sacroiliac pain, chest pain, stiffness and functional limitation and, at a peripheral level, is responsible for plantar fasciitis and Achilles tendinitis as the peripheral entheses most commonly described as affected, and is even implicated in the onychopathy shown by patients with psoriatic arthritis (PsA).5
In 1990, Bernard Amor for the first time explicitly collected the involvement of the peripheral entheses as a sign or symptom in the clinical history: “heel pain or pain of other well-defined enthesis” in their ranking criteria for the diagnosis of SpA. Later, in 1991, the European Group for the Study of SpA included enthesopathy again as one more “item” on their ranking criteria. However, until the development of modern imaging techniques, especially MRI and ultrasound, the diagnosis of enthesitis has been underestimated, mainly by the lack of sensitivity of the clinical examination for their detection. Thus, the involvement of the enthesis has not been properly included in either the evaluation or diagnosis of SpA until today. However, things are changing with the increasingly widespread practice of ultrasound among rheumatologists.6,7
Throughout this article, we will review how ultrasound opens new perspectives and possibilities in the field of SpA.
Definition and Overview of EnthesesEnthesis is defined as the region where a tendon, ligament, joint capsule, fascia or muscle attaches to the bone. It is a transition tissue whose function, besides being a soft tissue anchor, is to transfer the stress in these areas of attachment to the adjacent bone, and vice versa.
Histologically, Benjamin et al.8 distinguished 2 types of entheses in relation to the tissue they have in the anchorage zone: the fibrous enthesis, which binds metaphysis and diaphysis of long bones, and fibrocartilage enthesis, which joins epiphysis and apophysis of long bones, short bones of the hands and feet, and spine. The most common, and the ones of interest because they represent the target organ involvement in SpA, are fibrocartilaginous entheses, although it is unclear if the phenomenon of enthesitis only affects these.
The fibrocartilaginous entheses include 4 histologically distinct areas: (1) a fibrous zone composed of fibers of type 2 collagen and in which versican is the predominant extracellular matrix proteoglycan, (2) uncalcified fibrocartilage zone, in which aggrecan prevails as the extracellular matrix proteoglycan, (3) calcified fibrocartilage area and zone and (4) subchondral bone.
The calcified fibrocartilage zone (zone 2) of variable thickness and devoid of vessels, is theoretically where enthesis injury initially occurs and where the inflammation extends to the synovium and adjacent bone tissue.1 Nutrition is believed to come from the entheses vessels in the bone marrow, tendon fibrous region and through the adjacent fat and connective tissue.
In 2001, Benjamin and McGonagle introduced the concept of ‘entheseal body’,9,10 considering the enthesis as a body, formed by different tissues, and defining it as a “collection of related tissues in and around the enthesis, which serve the common function of dissipating tension.” This attempts to explain why the enthesis inflammation experienced by patients diagnosed with diffuse SpA is associated with changes in the surrounding tissues, both soft tissue (bursitis, edema of subcutaneous tissue) and bony unions (erosions, enthesophytes).11
The nail findings (onychopathy) displayed on the SpA are an extension of the changes that occur in the entheses of the distal interphalangeal joints into the matrix and nail bed, as has been shown by MRI.5,12
Furthermore, the synovial tissue of the bursa with the entheses forms the so-called “entheso-synovial complex”. Healthy entheses, in its fibrocartilage area, is avascular and presents low cell density and no inflammatory cells. Instead, the synovium is a vascularized structure and contains a resident population of immune cells and, therefore, the capacity for hyperplasia and immune response. Moreover, adipose tissue has a proprioceptive function by monitoring changes in the angle of insertion of the enthesis, an immune function, due to its high content of macrophages, and a nociceptive function, participating in the painful entheseal disease.
“Enthesopathy” and “Enthesitis”The term “enthesopathy” refers to the structural alteration of the enthesis by mechanical, traumatic, metabolic or even inflammatory causes, while the concept of ‘enthesitis’ is used when there is active inflammation of the entheses, as in SpA, although inflammatory changes may also occur in other, not necessarily inflammatory, conditions. Enthesitis is part of the clinical spectrum of SpA, in all subtypes: ankylosing spondylitis (AS), SpA, Reiter's syndrome, SpA associated with inflammatory bowel disease, juvenile SpA and, more recently, axial and peripheral SpA, and in almost all patients although, in the literature, the percentage of patients with clinical manifestations of enthesitis is variable (10%–60%).
Clinical Evaluation of EnthesisThe prevalence of enthesitis in SpA is not easy to determine for 2 main reasons: on one hand, the possible subclinical involvement of the entheses and, on the other, the diagnostic difficulty of the clinical examination, due to the absence of visible inflammatory signs. Still, indices have been developed to clinically assess entheses in patients with SpA.
There are 3 validated indices for AS (Mander enthesis Index [MEI], Maastricht Ankylosing Spondylitis enthesitis [MASES] and Major) and 2 indices validated for PsA (Glandman and Leeds). The MEI, published by Mander in 1987, evaluates 66 entheses, establishing a pressure pain, ranking making it difficult to apply when in clinical practice.13 Subsequently the MASES index was published, a simplification of the above, assessing the presence or absence of pain in 13 entheses. The Major index includes 12 entheses in their assessment: iliac, trochanter, epicondyles, epitrochlear, Achilles and plantar fascia. The Gladman index assesses eight enthesis: rotator cuff, anterior tibial tuberosity, Achilles and plantar fascia; Leeds includes 6 entheses in the evaluation: Achilles, medial femoral condyle and epicondyle. The examination is performed by applying constant pressure with the fingertips on the enthesis, which leads to loss of objectivity according to the pain threshold, considering it is not the same for each patient.
Evaluation of Enthesis Through ImagingThe reliability and accuracy of the clinical examination to assess entheses are not satisfactory, so imaging techniques have potential use in their objective assessment. X-rays and computed tomography only detect and evaluate structural bone changes that correspond to past episodes of activity or injury and do not inform us of the presence of inflammatory activity in enthesis at the time of examination. Thus, imaging tests such as MRI and, more recently, ultrasound, have been employed for the diagnosis of active disease in patients with SpA.
MRI has been used in SpA, mainly to assess axial involvement: cervical, dorsal, lumbar and sacroiliac joints. It allows early visualization of Romanus lesions in the spine and enthesitic interspinous and supraspinal ligament injuries, but mostly it has been validated in sacroiliitis. In the swollen entheses, what is detected by NMR is subcutaneous tissue or soft tissue edema (perienthesitic edema) and bone edema; less frequently it detects entheses edema through their connection between fibroblasts and collagen fibers in the fibrous part of the enthesis. For this reason, MRI is insensitive and nonspecific for assessing enthesitis.
However, considering that, until relatively recently, treatment options were limited in SpA, it was not necessary to develop a new imaging technique to assess disease activity and to assess response to treatment.
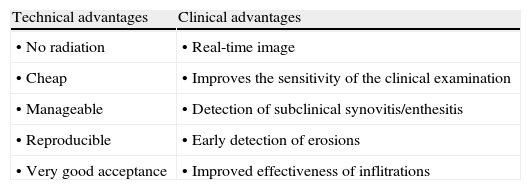
Ultrasound has a number of technical advantages; it does not emit radiation, it is economical, manageable, reproducible, has very good acceptance by patients and clinicians as well as some clinical advantages, because it offers a real-time image and the ability to evaluate multiple locations during the same examination, improves the sensitivity of the clinical examination in detecting synovitis and enthesitis and likewise improves the sensitivity of plain X-rays in detecting erosions. On the other hand, it is able to adequately guide aspirations and injections, increasing their effectiveness (Table 1). Among the limitations attributed to this method are the time spent although, with practice, this is significantly reduced, and the limited visualization of some locations.
Advantages of Ultrasound in the Examination of the Musculoskeletal System.
| Technical advantages | Clinical advantages |
| • No radiation | • Real-time image |
| • Cheap | • Improves the sensitivity of the clinical examination |
| • Manageable | • Detection of subclinical synovitis/enthesitis |
| • Reproducible | • Early detection of erosions |
| • Very good acceptance | • Improved effectiveness of inflitrations |
In SpA, in addition to assessing joint involvement, ultrasound is used to assess entheses14,15 and is also starting to be used to assess sacroiliac joints. Although its application in SpA is recent, it has already proven superior in terms of sensitivity to clinical examination for detecting enthesitis and16 has revealed a high frequency of abnormalities in asymptomatic patients with subclinical enthesitis,17 which makes it a potential tool for a good objective assessment of patients with SpA.
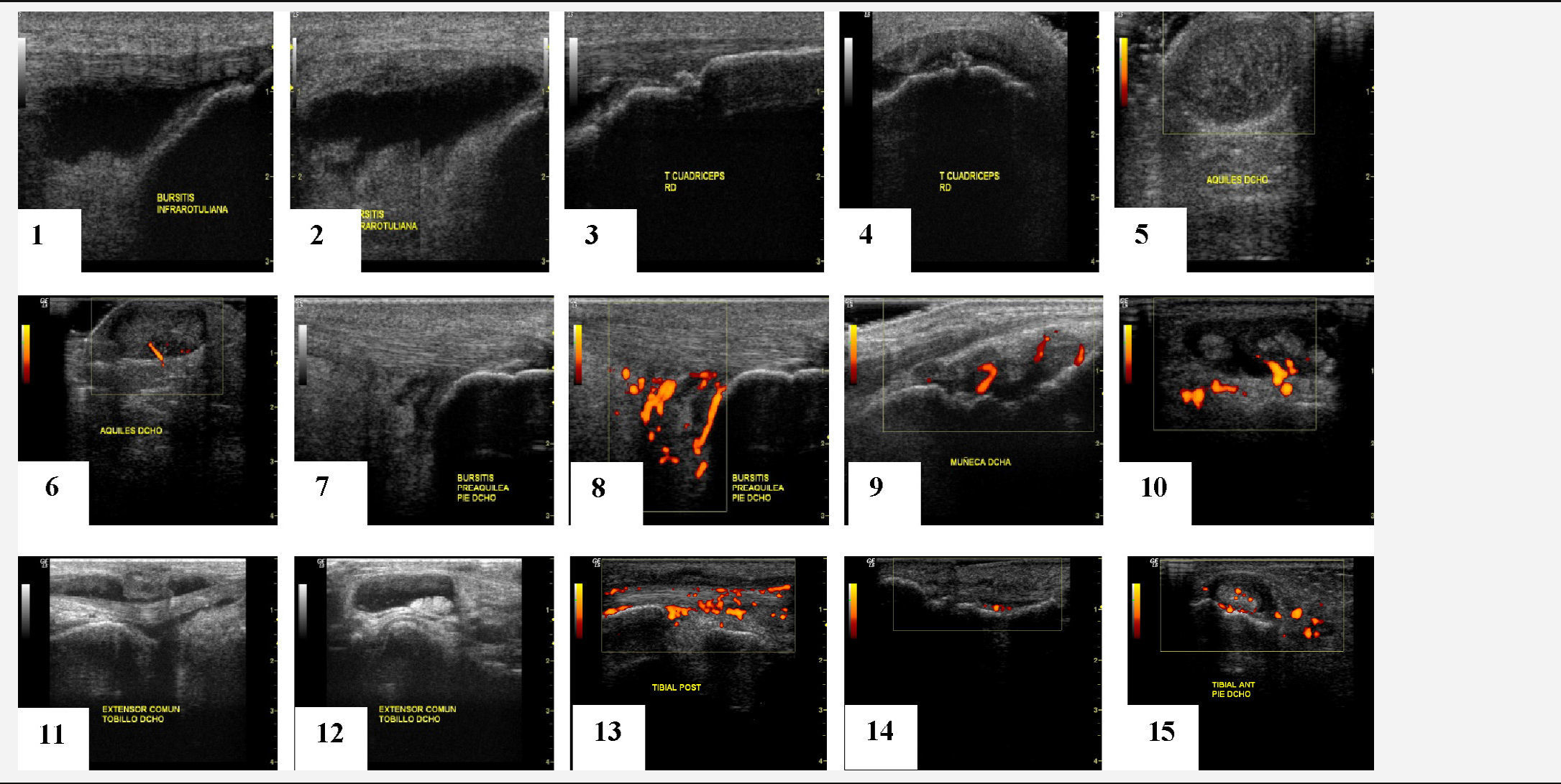
A variety of ultrasound findings in B (grayscale) mode have been described, in peripheral enthesitis of patients with SpA: bursitis, enthesophytes, loss of normal fibrillar echotexture, echogenicity loss, edema of subcutaneous tissue, thickening of the enthesis, bone erosions18,19 (Table 2), some of them considered activity lesions, as well as other chronic changes, although this classification of enthesis lesions has not yet been validated in any paper.
Moreover, the Doppler function allows for the detection of hyperemia or pathological vascularization in both the synovium and synovial sheath of tendons, bursae and entheses.20
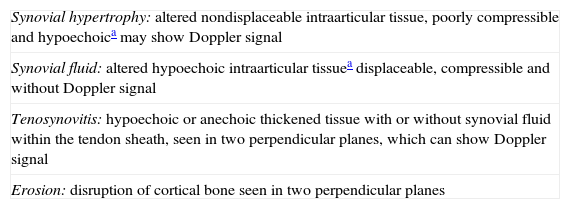
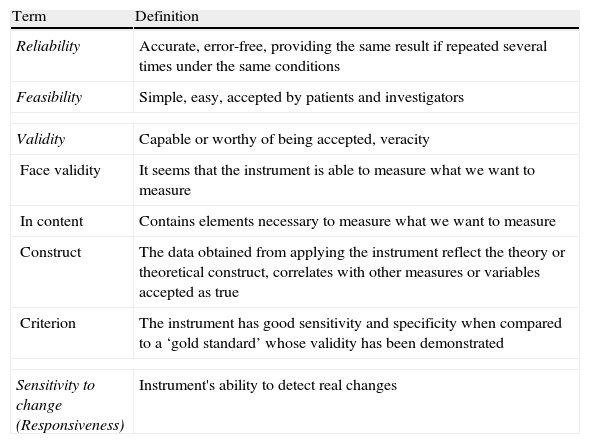
Evolution of Ultrasound in SpondyloarthritisIn order to perform a standardized assessment of joint ultrasound, it is essential to define and validate ultrasound concepts (Table 3). The Outcome Measures in Rheumatology Working Group (OMERACT) was created to identify, standardize and validate measurement tools, as well as clinically important definitions to be included in clinical trials in rheumatic diseases. Their approach is to apply the so-called ‘OMERACT filter’ based in turn on that described by Bombardier and Tugwell in 1982,21 which simplifies the methodology into 3 basic concepts: truth, discrimination and feasibility; the latter includes the concepts of reliability and sensitivity to change (Table 4).
Standardized Definitions of Joint Ultrasound (OMERACT 7).23
| Synovial hypertrophy: altered nondisplaceable intraarticular tissue, poorly compressible and hypoechoica may show Doppler signal |
| Synovial fluid: altered hypoechoic intraarticular tissuea displaceable, compressible and without Doppler signal |
| Tenosynovitis: hypoechoic or anechoic thickened tissue with or without synovial fluid within the tendon sheath, seen in two perpendicular planes, which can show Doppler signal |
| Erosion: disruption of cortical bone seen in two perpendicular planes |
Characteristics of Measuring Instruments.
| Term | Definition |
| Reliability | Accurate, error-free, providing the same result if repeated several times under the same conditions |
| Feasibility | Simple, easy, accepted by patients and investigators |
| Validity | Capable or worthy of being accepted, veracity |
| Face validity | It seems that the instrument is able to measure what we want to measure |
| In content | Contains elements necessary to measure what we want to measure |
| Construct | The data obtained from applying the instrument reflect the theory or theoretical construct, correlates with other measures or variables accepted as true |
| Criterion | The instrument has good sensitivity and specificity when compared to a ‘gold standard’ whose validity has been demonstrated |
| Sensitivity to change (Responsiveness) | Instrument's ability to detect real changes |
The application of ultrasound for assessing peripheral arthritis in patients with SpA adopted the same definition and validity concepts used in patients with RA (OMERACT 8), later also validated in SpA.22 Thus, in ultrasound findings of synovitis, tenosynovitis and erosions in SpA, the same definitions apply than for ultrasound in RA and other inflammatory arthritis (OMERACT 7)23 (Table 3).
However, in defining the entheseal involvement of patients with SpA, ultrasound has had to start from scratch and the existing evidence created in recent years has led to a progressive and steady acceleration of knowledge.
First, ultrasound has been shown to be a tool with good sensitivity and specificity for the diagnosis of enthesitis.16–18 There are enthesis studies on ultrasound since 1994,19 but starting in 2002 they began to increase in number and quality of publications. Above all, the description of the features of enthesitis significantly improved in the studies published since 2005, due to the technological advancement of ultrasound equipment, as well as the publication of the first definition of enthesopathy by the OMERACT group. Hypoechoic alterations (loss of normal fibrillar architecture) and/or thickening of the ligament or tendon at its bony insertion (which may occasionally contain hyperechoic foci suggestive of calcification), seen in two perpendicular planes, and which may show, or not, Doppler signal and/bone changes including enthesophytes, erosions and irregularities.
In addition to those described in this definition, there are other basic ultrasound lesions, such as bursitis and alteration of fibrocartilage, probably involved in the pathogenesis of the disease which have led to publications.24,25
Multiple studies have demonstrated the validity aspect of ultrasonography for assessing enthesitis,14–17,26 as it is also a technique capable of detecting various enthesis lesions occurring in patients with SpA (thickness, echostructure, edema, bursitis, erosions, enthesophytes, Doppler signal, tendon ruptures) and in different locations likely to be affected, demonstrating also its content validity.26
There are fewer studies that have demonstrated construct27–29 and criterion validity.30 Construct validity has been demonstrated obtaining a good correlation between ultrasound and clinical history and examination, both for chronic and acute enthesitis in patients with SpA.29 But, the gold standard for enthesitis is histology which, for obvious reasons, cannot be performed in patients. In an attempt to demonstrate criterion validity by correlating ultrasound with what actually happens histologically, McGonagle et al. compared the outcome of30 sonographically evaluating the Achilles entheses of patients with SpA with the histological findings of elderly cadavers, noting in both groups that erosions appear in the most proximal part of the entheses, where there is a greater amount of fibrocartilage and enthesophytes appear in the most distal portion, where the fibrocartilage amount is smaller. Another demonstrated feature has been moderate to excellent inter- and intraobserver reliability in the evaluation of Achilles enthesitis in patients with SpA, for all the entheseal findings described in the OMERACT definition except for hypoechoic lesions and cortical irregularities, probably due to lack of standardization of these lesions.18
There is emerging work demonstrating sensitivity to change of ultrasound in examining the progression of SpA, as well as in monitoring response to treatment as we discussed in the section on monitoring.
The first extensive description of ultrasound of enthesis involvement was performed by Lehtinen et al.19 in 1994, followed by the description given by Balint et al. in 200214; both authors describe the findings in B mode and conclude that there is a high frequency of incidental findings, i.e. patients diagnosed with SpA without entheseal symptoms yet who showed alterations in the enthesis evaluated by ultrasound or, which is the same, had a subclinical enthesopathy. But, although ultrasound B mode is useful for detecting enthesopathy, it is not so much in revealing active inflammation.
In 2003, D’Agostino et al. described and published a paper on the utility of Power Doppler function to evaluate hyperemia and neovascularization in the entheses, which is indicative of inflammatory activity. For these authors, in addition, the location of the Doppler signal is a specific finding of SpA, as evidenced by comparing patients with this condition to a control group that included patients with RA and patients with low back pain. Patients with SpA had Doppler signals at the enthesis insertion into cortical bone, whereas the control group presented a perientesis Doppler signal, especially of the retrocalcaneal bursa in patients with RA.15 However, Doppler of the enthesis has failed to demonstrate criterion validity in the absence of a ‘gold standard’ or feasible comparison considering that MRI has a low sensitivity for detecting edema in the enthesis and histology is not feasible for ethical reasons.
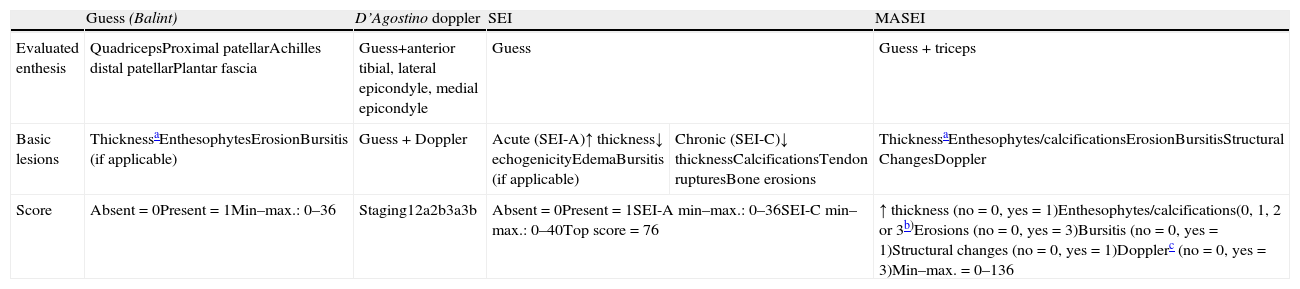
Indices for Sonographic Assessment of EnthesesThe enthesis ultrasound can be performed on a particular enthesis according to the area reported by the patient as painful in the history, or through a more comprehensive assessment examining several entheses. In the literature, various ultrasound entheseal indices are collected, some using only the B or grayscale mode and incorporating the latest Doppler function. Table 5 describes the main features of ultrasound indices used to assess entheses in patients with SpA.
Description of the Characteristics of Different Indices to Assess Ultrasound Enthesis.
| Guess (Balint) | D’Agostino doppler | SEI | MASEI | ||
| Evaluated enthesis | QuadricepsProximal patellarAchilles distal patellarPlantar fascia | Guess+anterior tibial, lateral epicondyle, medial epicondyle | Guess | Guess + triceps | |
| Basic lesions | ThicknessaEnthesophytesErosionBursitis (if applicable) | Guess + Doppler | Acute (SEI-A)↑ thickness↓ echogenicityEdemaBursitis (if applicable) | Chronic (SEI-C)↓ thicknessCalcificationsTendon rupturesBone erosions | ThicknessaEnthesophytes/calcificationsErosionBursitisStructural ChangesDoppler |
| Score | Absent=0Present=1Min–max.: 0–36 | Staging12a2b3a3b | Absent=0Present=1SEI-A min–max.: 0–36SEI-C min–max.: 0–40Top score=76 | ↑ thickness (no=0, yes = 1)Enthesophytes/calcifications(0, 1, 2 or 3b)Erosions (no=0, yes=3)Bursitis (no=0, yes=1)Structural changes (no=0, yes=1)Dopplerc (no=0, yes=3)Min–max.=0–136 | |
The first and most reproduced published study is called the Guess Index, developed by Balint et al.14 It evaluates four elementary lesions (thickness, enthesophytes, erosions and bursitis) bilaterally on 5 entheses of the lower extremity, using only B-mode demonstrating that ultrasound exceeds physical examination in sensitivity to assess enthesitis. D’Agostino et al. introduced the Doppler function in the assessment of entheses,15 noting that ultrasound is a useful technique to assess both elementary enthesis lesions, to detect inflammatory activity in patients with SpA. It establishes five evolutionary stages in the involvement of the enthesis (Table 6).
Ultrasound Staging Index by D’Agostino.
| 1 Vascularization in the cortical union without lesions in B mode |
| 2a Edema associated vascularization and/or decreased binding in cortical echogenicity in B mode |
| 2b findings in B mode of stage 2 without vascularization |
| 3a stage 2A + erosions or enthesis calcifications and optional associated bursitis |
| 3b stage 3 findings in B mode without vascularization |
Both indices (Guess and D’Agostino) are preliminary and pre-date the definition of enthesopathy proposed by OMERACT.23 The Spanish enthesitis index (SEI Index) is an extension of the elementary lesions of the Guess index, which also evaluates decreased echogenicity, tendon ruptures, decreased thickness and edema; seeks to identify lesions consistent with active inflammation (SEI-A) and chronicity (SEI-C), based on the opinion of the authors, and does not use Doppler16; correlations of this index with other outcome variables used in SpA are poor or do not reach significance, especially in the activity index.
The Madrid Sonographic enthesitis Index (MASEI Index) is the most complete so far and the only one based on the OMERACT definition of enthesopathy. It has demonstrated higher sensitivity (83.3%) and specificity (82.8%) in the diagnosis of SpA in patients who present a score ≥18.17
There is only one published work in which Doppler ultrasound evaluated through the MASES clinical index is performed, which assesses 13 entheses,31 but the results were not good; it showed that clinical examination was more sensitive than ultrasound in most entheses, except for Achilles enthesis, probably because for the rest of the enthesis evaluated by this index, ultrasound windows used were not standardized or were easily reproducible.
Although bursitis is not included in the OMERACT definition of enthesopathy, all published indices ultrasound searched for this lesion. However, none included fibrocartilage lesions as elemental alterations, probably limited by ultrasound equipment used in their evaluation and due to its recent description. This article also did not demonstrate a high sensitivity and specificity in the classification of patients with SpA.
Enthesis Ultrasound to Monitor Response to Biological TreatmentsAlthough the use of ultrasound in SpA lags behind its use in RA, it is proving valid for the diagnosis of enthesitis and some study results also demonstrate its validity for monitoring progress and response to treatment.
Therapeutic options are limited for enthesitis: NSAIDs, local injections and, more recently, tumor necrosis factor (TNF) antagonist drugs have shown efficacy as measured by clinical examination, MRI and ultrasound.
Some studies have evaluated the sensitivity to change of ultrasound in entheseal manifestations of patients with SpA treated with sulfasalazine, with no differences at 6–12 months of treatment, probably not for lack of discriminant validity of the technique, but ineffectiveness of the drug for this condition.32,33
In another recent study in which the sensitivity to change of ultrasonography in Achilles enthesitis (grayscale and power Doppler signal) of AS patients treated with an TNF antagonist drug (etanercept, infliximab or adalimumab) was evaluated, all ultrasound findings improved after 2 months of anti-TNF therapy,34 although the results are poor.
But the best study to evaluate the response to treatment of various entheseal lesions is probably that published by Naredo et al.35 It evaluated, on one hand, lesions considered inflammatory (hypoechogenicity, thickening, power Doppler signal) and, on the other hand, structural lesions considered due to damage or chronic (enthesophytes and erosions). The described inflammatory lesions as well as the bursitis adjacent to the enthesis showed significant improvement after 6 months of anti-TNF therapy (infliximab, etanercept or adalimumab), while chronic lesions worsened despite anti-TNF therapy.
However, a study published subsequently demonstrated that entheseal erosions in patients with SpA36 are reversible and therefore should not be considered as a chronic structural lesion in these patients. In addition, ultrasound demonstrated sensitivity to change and therefore it is a useful tool for assessing Achilles enthesitis erosions in patients with SpA.
In conclusion, we can say that enthesitis is a key event in the clinical expression of SpA, but the physical exploration of the entheses lacks sensitivity, accuracy and reliability, which is a clear deficiency in the clinical assessment of these patients. For this reason, the use of imaging techniques to increase their accuracy and detect subclinical enthesitis becomes necessary. In this sense, ultrasound is gaining on MRI, mainly not only for its technical advantages, but also because it is demonstrating reliability, validity and, more recently, sensitivity to change. Much has been advanced and in a relatively short time, but for ultrasound to be useful in clinical practice it is necessary to validate and agree on entheseal ultrasound indices.
Ethical ResponsibilitiesProtection of persons and animalsThe authors state that no experiments were performed on persons or animals for this study.
Data confidentialityThe authors state that they have followed their workplace protocols regarding the publication of patient data and all patients included in the study have received enough information and have given their written informed consent to participate in the study.
Right to privacy and informed consentThe authors state that they have obtained informed consent from patients and/or subjects referred to in this article. This document is in the possession of the corresponding author.
Conflict of InterestThis article summarizes the final work of the first author in the Master of Spondylarthritis of the European University of Madrid, sponsored by Abbvie.
Please cite this article as: Mata Arnaiz MC, de Miguel Mendieta E. Utilidad de la ecografía en la evaluación de las entesis periféricas en las espondiloartritis. Reumatol Clin. 2014;10:113–119.