Patients with rheumatoid arthritis (RA) are at increased risk of infection compared to healthy individuals. The increased risk may be associated with the underlying disease, comorbidities, and immunosuppressive therapy required to control RA activity.
In several recent studies, influenza, pneumococcal, and hepatitis B vaccines administered to RA patients were reported to be safe and serologically effective.
However, several lines of evidence suggest a possible aberrant immunologic response following vaccination due to the compromised immunity of these patients. Therefore, vaccination of RA patients prior to immunosuppressive treatment may serve as an alternative prophylactic approach and should be considered for future investigation.
Besides, prophylactic health measures should be taken to avoid latent chronic infections as tuberculosis and hepatitis B, during therapy with biological agents.
Los pacientes con artritis reumatoide (AR) tienen un riesgo incrementado de padecer infecciones respecto a la población sana. Principalmente se debe a la enfermedad en sí, a la comorbilidad y al tratamiento inmunosupresor.
La evidencia clínica demuestra que la administración de las vacunas frente al neumococo y los virus influenza y hepatitis B en pacientes con artritis reumatoide no empeora la actividad de la enfermedad, y que éstas son eficaces, aunque parece que la respuesta inmune se ve reducida por la propia enfermedad y el tratamiento con FAME y biológicos. Por ello, es aconsejable vacunar a los pacientes al diagnóstico, antes de iniciar el tratamiento con inmunosupresores.
También, existen y se deben tomar medidas profilácticas para evitar la reactivación de infecciones latentes crónicas como la tuberculosis y la hepatitis B durante el tratamiento con biológicos.
It is estimated that patients with rheumatoid arthritis (RA) have twice the risk for infections for the stock sana.1 This increased risk is due in part to the disease itself, since poor prognosis factors have been identified as predictors of infection and other associated comorbidity (diabetes, alcohol, tobacco use, chronic obstructive pulmonary disease)2 and treatment with immunosuppressive drugs.3,4
So far, recommendations for the management of RA have determined that these patients should be vaccinated against pneumococcal disease, influenza, and hepatitis B.5 For other vaccines, they tend follow the same directions as for the healthy population, taking into account that the administration of live vaccines is contraindicated.
RA patients also have an increased risk of primary infection and are more likely to have reactivation of latent chronic infections such as tuberculosis (TB) or hepatitis B. Taken together, these infections involve an increase in both morbidity and mortality, hence making preventive behaviors such as vaccination and chemoprophylaxis essential.
However, although vaccines have proven effective in this group of immunosuppressed patients, they are little used today. It is estimated that their use is limited to 40%, while that of other preventive measures such as those directed against cardiovascular risk factors reaches 90%. This is mainly due to a lack of awareness by health professionals and the distrust generated by vaccines with respect to two fundamental aspects, safety and efficacy.6
Regarding safety, we must bear in mind that live vaccines are contraindicated in patients with immunosuppressive therapy. Also caution should be exercised when managing these patients with live-oral vaccine against poliomyelitis7 and against rotavirus,8 since it has been shown that these viruses are excreted in the feces after vaccination for a few days and could infect a patient with RA.
Regarding the use of inactivated vaccines in patients with RA, the concern for safety stems from a case series that suggest a clinical deterioration of patients after vaccination and even vaccines working as triggers for the disease.9,10 However, after several studies have shown no causal link between vaccines and worsening of the disease, expressed by clinical (tender joint, swollen joint count, health assessment questionnaire, visual analogue pain scale of the patient) and laboratory (CRP and ESR) parameters associated with RA activity. Thus, the evidence indicates that hepatitis B vaccine and pneumococcal vaccine can be administered as a grade B recommendation11,12 as well as the current influenza A vaccine.13–15
On the other hand, to evaluate the efficacy of vaccines, the right thing to do would be to conduct studies that measured the number of infections averted after vaccination. However, these are so far unavailable, and the immune response is used as a substitute for efficiency, which is determined by two parameters: immunogenicity (ability to respond to the antigen, and therefore produce an increase of specific antibodies) and protection (antibody level above a certain theoretical limit beyond which the patient is supposed to be protected). The objective of this review is to update public awareness of preventive measures such as vaccination and chemoprophylaxis in patients with RA and from it establishes a vaccination schedule and useful recommendations for clinical practice.
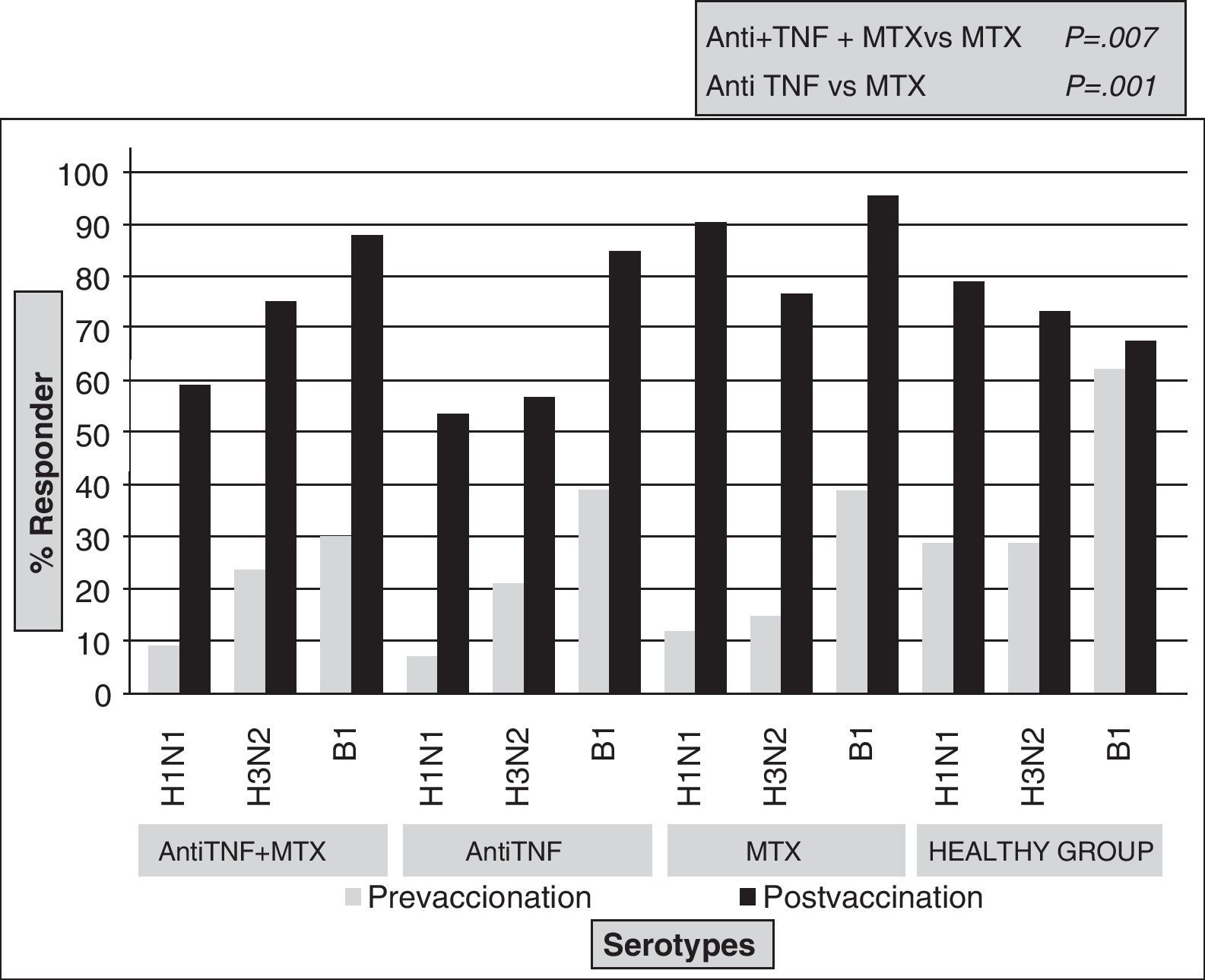
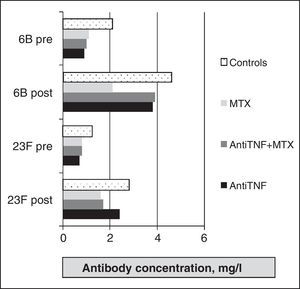
Vaccine Against Influenza VirusIt is an inactivated vaccine protein consisting of two strains of antigen, A and a B that change annually as recommended by the World Health Organization. Several trials have evaluated its efficacy in patients with RA. Formin et al.13 compared the immune response to influenza vaccine between a healthy control group and a group of RA patients with different treatments (DMARDs, etanercept or infliximab). In both groups, specific antibodies against strain A increased significantly, while the increase for strain B was higher in the healthy group (P<.05). In terms of safety, there was no statistically significant differences between groups for strain A, however, the protective response to strain B was achieved in 67% of RA patients versus 87% in healthy controls (P<.05). A relevant fact was that the percentage of respondents in the RA group was the same regardless of the treatment. Later,16 a study compared the response to influenza and pneumococcal vaccines between healthy controls and three groups of patients with RA (anti-TNF [infliximab or etanercept], methotrexate and combination therapy). An increase in antibody levels after vaccination for all strains in all groups was seen, but the protective response was significantly higher in the methotrexate monotherapy group compared with anti-TNF treatment (Fig. 1). However, these results differed in the study by Kaine et al.,17 in which the percentage of respondents in both groups (placebo±DMARD versus adalimumab±DMARD) was similar and the immune response to the vaccine did not differ by the use of concomitant DMARDs.
Furthermore, the effectiveness of the vaccine against influenza in patients with RA treated with rituximab has also been evaluated. The first study18 determined the response to the vaccine in RA patients and patients treated with DMARDs and rituximab. It was noted that the proportion of responders for strain B was lower in the rituximab group (21% vs 67%, P=.006), while the percentage of respondents for strain A was similar in both groups. Recently, the results of a study14 analyzing the response to the vaccine in RA patients treated with rituximab (n=23), methotrexate (n=20), and healthy (n=29) have been published. The Rituximab group was divided into two subgroups, one that received the vaccine 4–8 weeks after the last dose of rituximab and another who was given it 6–10 months after treatment. After vaccination, specific antibody titers for all three strains increased significantly in the healthy group and those with methotrexate, however, there were no significant increases in antibody in the rituximab group. On the other hand, the protection achieved for all strains in healthy controls and strain A in the methotrexate group was more frequent in the rituximab group; this difference was statistically significant. In the latter group, only 26% of patients achieved protective immunity, the majority belonging to the subgroup with a greater time difference between the administration of rituximab and the vaccine (6–10 months).
Furthermore, no correlation was found between serum level of B cells, the initial levels of antibodies, the immunogenicity and safety in patients with rituximab. The discrepancy between the results of that study and the first could be explained by the greater difference in time between rituximab treatment and vaccination in the study by Oren et al.18
In patients treated with abatacept, there are no data on safety and efficacy of the vaccine. In the case of tocilizumab, the evidence is very limited.19 There is a study comparing the response to vaccination against influenza in RA patients treated with tocilizumab and those obtained in patients with anti-TNF or DMARD, which found no statistically significant differences in the percentages of patients responding to each strain.
In summary, it has been shown that the influenza vaccine is safe in patients with RA and that the immune response in these patients may be lower than in the healthy population. Anti-TNF therapy and rituximab were associated with less protection against infection.
Pneumococcal VaccineThere are two types of pneumococcal vaccine: the polysaccharide-based, which contains 23 serotypes that cause 88% of pneumococcal infections, and protein-based (heptavalent and 13-valent), which is only indicated in children under 5 years and is more immunogenic but has not been used so far in patients with RA.
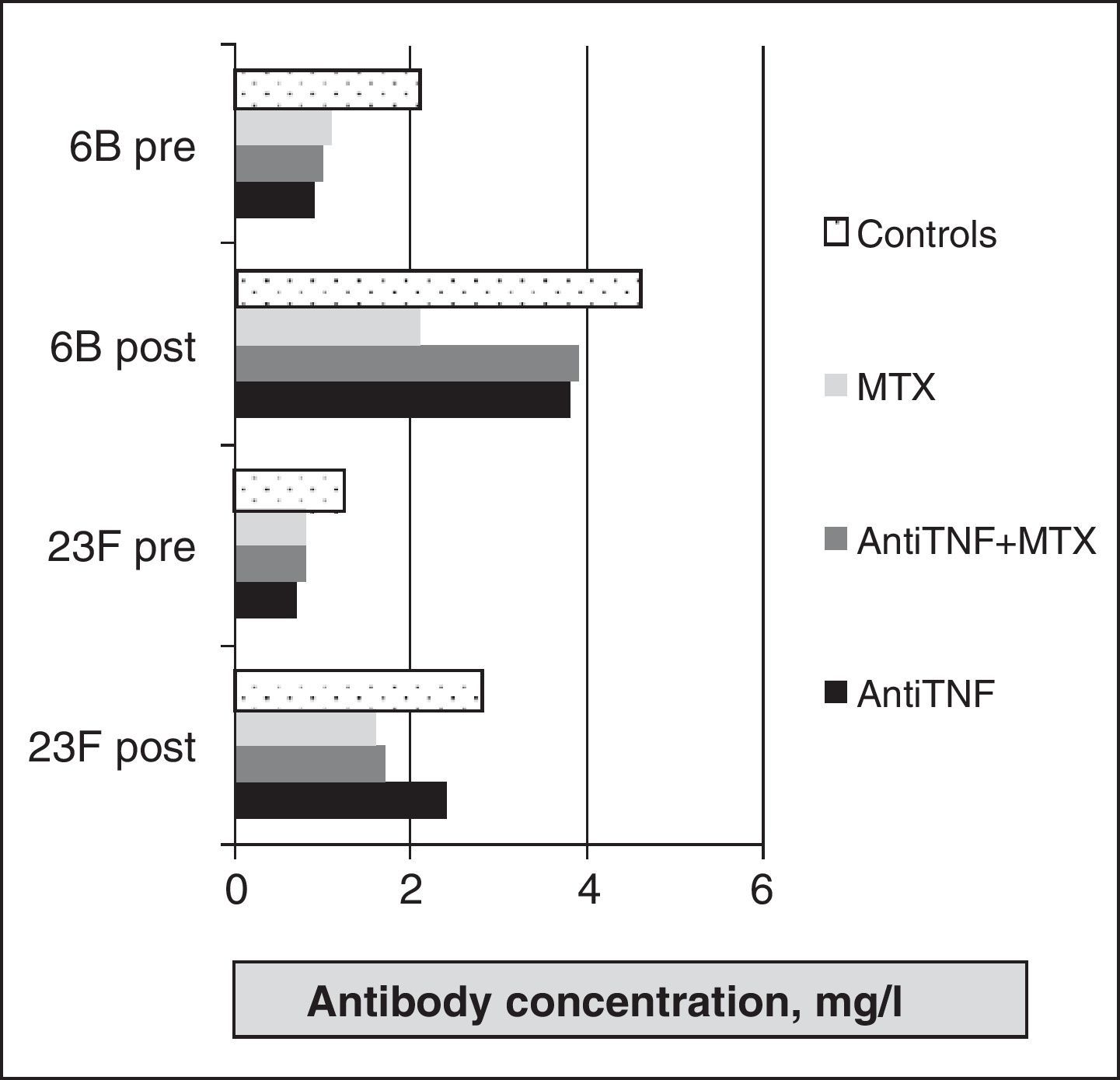
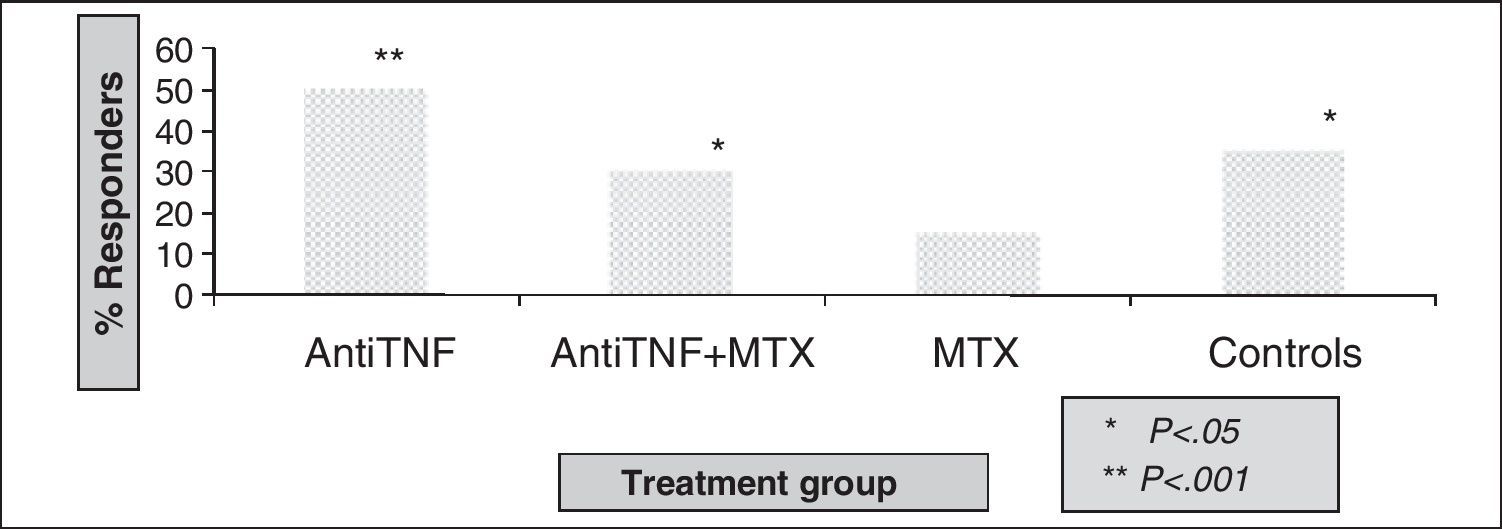
As for the evaluation of polysaccharide vaccine efficacy in RA, a first study of 12 compared the response in patients with RA treated with DMARDs (mostly methotrexate) and healthy controls. RA patients had significant increases in the concentration of specific antibodies to all serotypes studied. The percentage of responders was higher in the healthy group, but a statistically significant difference was found only in the 7F serotype. Treatment with prednisone, methotrexate, hydroxychloroquine, or azathioprine did not influence the response. Kapetanovic et al.16 analyzed the immune response in 149 patients with RA (anti-TNF [infliximab or etanercept], methotrexate, and combination therapy) and 18 healthy controls. Antibodies after vaccination were significantly increased compared to prevaccination levels for all treatment groups (Fig. 2), although the protective response was lower in the methotrexate group, with statistically significant differences compared to other groups (Fig. 3).
Another study20 confirms previous findings regarding the effectiveness of the vaccine in patients with infliximab, and evaluates the response in 70 patients assigned to three treatment groups (infliximab 3mg/kg plus methotrexate, infliximab 6mg/kg plus methotrexate, and placebo plus methotrexate). There was no difference in the response to the vaccine among the three groups.
On the other hand, Kaine et al.17 found no difference between the percentage of patients protected in the adalimumab and placebo groups. They determined that DMARD and protective titers before vaccination significantly reduced the immune response. Recently,21 a study compared the response in a group of patients with RA treated with rituximab, with or without methotrexate, and a group of RA treated with methotrexate alone. After administration of the vaccine (28 weeks after treatment with rituximab), they found that response rates in the rituximab group were lower compared to methotrexate for all serotypes. They highlight the fact that serum levels of B cells did not predict the response in the rituximab group and the only significant predictor identified was the concentration of IgG2 at the time of immunization, so that for every increase of 1mg/dl, IgG2 response to the vaccine increased by 1%.
The evidence with other biological therapies is very limited. In patients with RA treated with abatacept, the immune response to pneumococcal vaccine was evaluated in the study ARRIVE22 (no control group) and found that abatacept does not completely inhibit the immune response to the vaccine. In patients treated with tocilizumab there is a study23 in which all patients showed a protective response following vaccination.
In conclusion, the safety of pneumococcal vaccine in RA patients has been demonstrated, and as for its effectiveness, it seems limited by both the disease itself and by treatment with DMARDs, rituximab, and abatacept.
Hepatitis B Vaccine and ProphylaxisAll patients with RA should undergo a serology for hepatitis B and C during the diagnosis stage, or before starting treatment with immunosuppressants, since, according to the methotrexate and leflunomide insert, one must have great caution in treating patients with liver disease. Biological drugs are contraindicated in patients infected with hepatitis B virus (HBV). Serology being negative, the patient should be vaccinated prior to the start of appropriate treatment. It has been observed that the administration of this vaccine in patients with RA is safe.11 To date, there are no studies directly comparing the immune response after vaccination against HBV in patients with RA and healthy controls. It is estimated that around 85% of healthy individuals respond to the vaccine, while a study11 conducted in patients with RA treated with DMARDs observed protective immunization in 68% of them. Treatment with methotrexate did not affect the response rate, however, it has been shown that treatment with etanercept in combination with methotrexate or as monotherapy significantly reduces the response to vaccine.24 It is necessary to conduct further studies evaluating the efficacy of this vaccine in RA patients treated with other biologics.
If the patient has acute or chronic active HBV, no immunosuppressive therapy should be initiated in any case and the patient should be sent to the hepatologist. However, in patients with chronic inactive disease or hidden carriers, despite being contraindicated, biologics are used in some cases due to high disease activity. The existing data on hepatitis B patients with rheumatic disease and immunosuppressive therapy for a long period of time are limited to isolated cases or small series of cases. According to these data, it seems safe to start treatment with anti-TNF after a month of antiviral treatment (lamivudine, entecavir), which must be maintained while patients receive anti-TNF therapy, but must always be treated in agreement with the hepatologist. During this period, liver serology, viral load, and serum transaminase levels should be monitored every 4–8 weeks to detect activation25 of infection. For patients with occult infection, the guidelines are less clear. It seems that the risk of reactivation is low, but two cases have been reported during treatment with etanercept and infliximab.26,27 Kim et al.28 showed a low rate of reactivation of infection in patients with occult infection, so in these cases it appears sufficient to monitor serum transaminase levels, serology, and viral load every 4–8 weeks.
With respect to other biological drugs, two cases of patients with hepatitis B infection treated with rituximab, one patient with RA and one with ankylosing spondylitis, have been published in recent years. There are no published data regarding other biological therapies.
Herpes VaccineHerpes zoster infection is the most common viral complication in patients with RA treated with anti-TNF.29 Immunosuppression increases the complications associated with infection such as the risk of severe rash, visceral affection, or death; however, the vaccine against herpes zoster virus is composed of live attenuated virus and therefore represents a risk for infection in immunosuppressed patients. Hence, there was a controversy over the appropriateness of use in patients with RA treated with immunosuppressants.
The task group of the American College of Rheumatology5 discouraged his administration in this group of patients. In contrast, the CDC Advisory Practices30 Committee on Immunization recommended vaccinating patients with RA over age 60 who are being treated with prednisone (<20mg/d), methotrexate (<0.4mg/kg/week) or azathioprine (<3mg/kg/day) or will start treatment with biologics (at least 15 days before the onset). All guidelines for the management of RA contraindicated vaccination in patients who began treatment with biologics.
Chemoprophylaxis of Latent Tuberculosis InfectionThere is evidence of an increased incidence of TB in patients treated with anti-TNF compared to the general population and people with RA with other treatments. Therefore, national31 recommendations stipulate that every patient with RA who is a candidate to initiate biological therapy patients has to undergo a Mantoux and chest x-ray. So far, the tuberculin skin test remains the gold standard test against, compared to QuantiFERON, to detect latent TB. Positive tests (induration greater than 5mm) should start prophylaxis with isoniazid for 9 months. One month after the start of prophylactic treatment, it seems safe to initiate biological therapy.
It has been reported that between 8% and 11% of patients32,33 became positive for the Mantoux during the first year of treatment with anti-TNF, so one may want to repeat the test regularly (annually) while undergoing treatment, although it has not been established how often to perform the test.
ConclusionsPatients with RA have increased risk of infections, associated to the disease itself, comorbidities, and immunosuppressive treatment.
This justifies the importance of preventive behavior such as vaccines and chemoprophylaxis. It has been shown that the vaccines against influenza, pneumococcus, and hepatitis B are safe in these patients. The effectiveness of each vaccine has been evaluated with tests, in most cases for studies with small, non-randomized groups of patients receiving different treatments, and different determinations of immune response, so that direct comparison is not appropriate. According to the results obtained, we can say that the percentage of responders to the vaccine against influenza is reduced by RA itself, as well as anti-TNF therapy and rituximab. Furthermore, the effectiveness of the pneumococcal vaccine appears to be affected by RA and by treatment with methotrexate, rituximab, and abatacept. In the case of hepatitis B vaccine, the protective response is lower in patients with RA and anti-TNF therapy. After reviewing the information available, a recommended immunization schedule in patients with RA should be:
- -
Annual influenza vaccine.
- -
Pneumococcal vaccine (Repeat every 3–5 years).
- -
Hepatitis B vaccine.
As has been proved, immunosuppressive treatments can reduce the effectiveness of the vaccine, and vaccination is recommended for patients when the diagnosis is made, because even first-line drugs such as methotrexate reduce immunization.
Clinical trials evaluating the efficacy and safety of vaccines in patients with RA treated with biological are being conducted; their results will expand the clinical evidence of the recommendations contained in this review.
Conflict of InterestThe authors have no conflict of interest.
Please cite this article as: Garrido López BC, et al. Vacunas y quimioprofilaxis en artritis reumatoide: ¿podría plantearse un calendario de vacunación? Reumatol Clin. 2001;7(6):412–16.