Patients with rheumatoid arthritis (RA) treated with disease-modifying antirheumatic drugs are susceptible to severe infections such as leishmaniasis. As L. infantum is endemic in the Mediterranean region, it is necessary to rule this infectious process out in any RA patient presenting with fever and pancytopenia. An early diagnosis based on a high suspicion can prevent a fatal outcome.
Los pacientes con artritis reumatoide (AR) tratados con fármacos modificadores del curso de la enfermedad están expuestos a desarrollar infecciones potencialmente graves como la leishmaniasis. L. infantum es endémica en el Mediterráneo, hecho que obliga ante un paciente con AR que presenta fiebre y pancitopenia, a descartar este proceso. Un diagnóstico de sospecha precoz, puede evitar un curso y pronóstico fatal.
The spectrum of the possible comorbidities associated with rheumatoid arthritis (RA) per se includes not only infections, but those attributable to the use of the conventional treatment of those infections. Their high incidence has been reported both for glucocorticoids and immunosuppressive agents, and with the use of biological therapy (BT). Among the options, the drug most widely utilized as first-line treatment is methotrexate (MTX), which enables a better control of the disease, although it can be accompanied by serious adverse effects such as hematotoxicity, which can develop in up to 15% of RA patients.1
To this day, the scope of reported opportunistic infections is very wide, ranging from primary infection by Nocardia or Pneumocystis carinii and fungal infections by Candida, Aspergillus, Cryptococcus or Histoplasma, to viral infections, mainly by herpes zoster. Surprisingly, although it is documented, there are few cases of Leishmania infection.2–4 Given the low incidence of visceral leishmaniasis (VL) in the Mediterranean region, there has been no recommendation for screening prior to initiating BT, and periodical serological surveillance of these patients is proposed only if they live in areas in which the frequency is higher.5,6
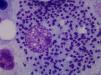
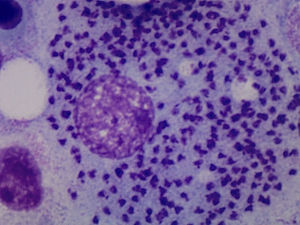
Case ReportThe patient was a 64-year-old woman who had undergone bilateral hip arthroplasty, and had been diagnosed with RA in 2002 because of polyarthritis, which was symmetrical, erosive and seropositive (positive for rheumatoid factor and anti-cyclic citrullinated peptide). She progressively developed asymmetrical arthritis, which affected large and small joints. As background therapy for her disease she received 15mg/week of oral MTX, 5mg/week of folic acid, indomethacin at 25mg/8h and low-dose prednisone. She had no significant epidemiological history. In 2012, she had begun to note considerable weakness, with episodes of spiking fever. At the time of the present evaluation, it had started 2 months earlier and was accompanied by pancytopenia (leukocytes: 3200 [lymphocytes: 1100], hemoglobin: 9g/L, platelets: 91,000), observed in a routine analysis. She was admitted to the hospital to be studied. The physical examination revealed mucocutaneous pallor and there were no signs of lymphadenopathy, and the review of systems produced no new findings. Treatment with MTX was discontinued due to the suspicion of hematotoxicity. Abdominal ultrasound and computed tomography of abdomen and chest established the presence of splenomegaly. Serology for human immunodeficiency virus and hepatitis B and C viruses was negative, and a bone marrow aspirate (BMA) confirmed marrow cellularity preservation, and diffuse intra- and extracellular involvement of leishmaniasis (Fig. 1). Once the diagnosis of VL had been established, treatment with liposomal amphotericin B was begun at a dose of 3mg/kg body weight until the patient had received 10 doses. After 10 weeks, she was afebrile and her blood count began to improve gradually until it was normal. At the present time, she is asymptomatic and is taking hydroxychloroquine, and good clinical control has been achieved in the management of RA.
DiscussionRheumatoid arthritis patients can develop a wide range of associated extra-articular comorbidities; of these, hematological disorders and infections account for a high percentage of cases, and, thus, the differential diagnosis is very extensive. Check-ups and follow-up visits of patients with RA are employed to monitor the analytical profile—hematological and biochemical—in accordance with recommended clinical practice guidelines.7 The study of a single cytopenia or of pancytopenia should include the complications of the disease itself, the associated drug toxicity and possible nosocomial infections; in addition, hematological conditions should also be ruled out.
Leishmaniasis is understood to be a set of syndromes caused by infection of the protozoan of the genus Leishmania, which is widely extended in tropical regions, whereas L. infantum causes a disease that is endemic in the Mediterranean. It has three clinical profiles: cutaneous, mucosal and visceral. The latter can be fatal in the absence of treatment. It is transmitted by the bite of the phlebotomine sandfly. Depending on the Leishmania species, the genus of the transmitting insect and the geographic region, the main reservoirs are dogs, rodents and persons. The onset of VL can be silent or, in contrast, full-blown (kala-azar), with its characteristic pentad: prolonged fever, weight loss, hepatosplenomegaly, pancytopenia and hypergammaglobulinemia. At some point, the subclinical disease can become symptomatic in patients who receive immunosuppressive agents like glucocorticoids, MTX or BT. There are three ways to establish the diagnosis of leishmaniasis: clinical, parasitic and immunological. The parasitological diagnosis should be confirmed by the observation of the protozoan in a tissue or smear. Should there be any doubt about the diagnosis, the BMA provides satisfactory results. The differential diagnosis of full-blown VL should include malignant hematological and lymphatic disorders, as well as infectious diseases like malaria. At the present time, the treatment of choice of VL in developed countries is liposomal amphotericin B.
In a review of the literature, it is noteworthy that there are fewer cases of patients with RA being treated with MTX than those receiving anti-tumor necrosis factor (TNF) agents who develop Leishmania infection. We have yet to know why. These patients do not seem to have any common factor predisposing them to infection. Methotrexate reduces the cell immune response, inhibits neutrophil chemotaxis and T-cell proliferation, and has been related to the development of infections by opportunistic pathogens, among which we find Leishmania.
Patients with RA being treated with MTX have a greater predisposition to develop VL than the general population, possibly due to immunological changes related to the underlying disease and the treatment they receive.
Tumor necrosis factor is a cytokine involved in the immune-mediated response to infection, especially against intracellular pathogens. The use of anti-TNF drugs in RA is growing, and their utility is clearly related to an increase in the number of opportunistic infections in these patients.
The diagnosis of VL should be considered in patients when there is a suspicion of infection if they are receiving MTX to treat an inflammatory rheumatic disease and live in regions in which leishmaniasis is endemic.
The importance of the early diagnosis, established during the follow-up visits in relation to the underlying disease, made it possible for us to detect VL in our patient. The prompt initiation of a specific treatment is fundamental in order to improve the prognosis of a potentially serious disease. With this in mind, we must not forget that, although uncommon, leishmaniasis is endemic in the Mediterranean.
ConclusionThe current management of RA patients is very satisfactory.8 Improvements in monitoring and the identification of new therapeutic targets have resulted in high survival rates. The prognosis is likewise more favorable; however, the same therapies that, on occasion, lead to remission, also have certain additional risks.9,10
The present report describes an infectious process that developed in the course of RA, partially due to the associated susceptibility to chronic inflammatory disease, together with the added risk conferred by immunosuppressive therapies like MTX. The study of comorbidities of RA should emphasize infections by the usual pathogens, without overlooking those produced by uncommon opportunistic microorganisms like Leishmania.
Ethical DisclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of InterestThe authors declare they have no conflicts of interest.
Please cite this article as: Reina D, Cerdà D, Güell E, Martínez Montauti J, Pineda A, Corominas H. Kala-azar en un paciente con artritis reumatoide tratada con metotrexato. Reumatol Clin. 2017;13:354–356.