Data reporting mortality in rheumatic diseases vary widely. The objective of this systematic review and meta-analysis of published data is to provide an accurate overview of the current risk of mortality in rheumatic diseases.
MethodsSystematic review and meta-analysis of published studies identified by a sensitive search using free text and MeSH synonyms of “mortality” and of “rheumatic diseases”, in general and by specific diagnoses. Eligibility criteria were (1) study population with rheumatoid arthritis, systemic lupus erythemathosus, systemic sclerosis, vasculitis, osteoarthritis, osteoporosis, dermatomyositis, or spondyloarthritis; (2) outcome of interest mortality, reported as an standardized mortality ratio (SMR), or easily calculated from data reported; and (3) cohorts or longitudinal observational studies. Assessment of risk of bias relied on the New Castle-Ottawa scale for cohorts; only moderate to high quality studies were included. Separate meta-SMRs were calculated for specific diagnoses. Heterogeneity was studied with meta-regression.
ResultsA total of 32 studies were included, none in spondyloarthritis or osteoarthritis. The overall pooled SMR was 2.03 (95% confidence interval (CI) 1.79–2.29), ranging from 1.36 in psoriatic arthritis to 4.80 in vasculitis. The largest individual overall SMR came from studies on inflammatory diseases, and the specific SMR were very high for infections and pulmonary events. Heterogeneity between studies was large; however, the analysis of such heterogeneity within diseases did not provide any association with the collected variables.
ConclusionsBased on our results and on the good quality of the included studies, we can conclude that rheumatic diseases increase in general the risk of death, and especially inflammatory diseases.
Losdatos de mortalidad en las enfermedades reumáticas es muy variable. El objetivo de esta revisión sistemática y meta-análisis de datos publicados es proporcionar una visión general más precisa del riesgo actual y mortalidad en las enfermedades reumáticas.
MétodosRevisión sistemática y meta-análisis de estudios publicados e identificados por una búsqueda utilizando texto libre y sinónimos MeSH de “mortalidad” y “enfermedades reumáticas”, en general, y por diagnósticos específicos. Los criterios de selección fueron: 1) población de estudio con artritis reumatoide, lupus eritematoso sistémico, esclerosis sistémica, vasculitis, osteoartritis, osteoporosis, dermatomiositis, o espondiloartritis, 2) resultados de mortalidad de interés, reportados como SMR, o fácilmente calculados a partir de los datos comunicados, y 3) cohortes longitudinales o estudios observacionales. Evaluación del riesgo de sesgo basado en la escala de cohortes New Castle-Ottawa, sólo estudios de moderada a alta calidad fueron incluidos. Se calculó meta-SMR para diagnósticos específicos. La heterogeneidad se estudió con meta-regresión.
ResultadosUn total de 32 estudios fueron incluidos, ninguno de espondiloartritis o osteoartritis. El SMR general combinada fue 2,03 (IC 95%: 1,79 a 2,29), desde 1,36 en la artritis psoriásica a 4,80 en las vasculitis. El mayor SMR general individual fue a partir de estudios sobre enfermedades inflamatorias, y SMR específicos fueron muy altos para las infecciones y reacciones pulmonares. La heterogeneidad entre los estudios era grande, sin embargo, el análisis de heterogeneidad dentro de las enfermedades no presentó ninguna asociación con las variables recogidas.
ConclusionesEn base a los resultados y la buena calidad de los estudios incluidos, se puede concluir que las enfermedades reumáticas en general aumentan el riesgo de muerte, y especialmente las enfermedades inflamatorias.
Mortality is a reliable indicator of illness severity.1 Rheumatic diseases are frequent chronic diseases with a great burden from disability and complications that ultimately lead to large losses of quality of life and cumbersome dependency, with demonstrated worse quality of life than other more serious diseases2; albeit they are considered benign diseases as a whole, because they are perceived to have a low mortality. Nevertheless, some rheumatic diseases may be mortal, especially those under the category of inflammatory diseases,3,4 and others may seem less deadly than they actually are. The recognition of the gradient of death risk among rheumatic diseases and of a decreased life expectancy in general, may subsequently lead to an acknowledgement of rheumatic diseases as relevant ones, with implications in health policies as well as in research budgets; despite the fact that those who manage rheumatic diseases, and rheumatic patients in general, find some very common health states in these diseases worse than death.5
An adequate risk assessment of patients during follow-up can help rheumatologist to improve the management in this rheumatic diseases, including an assessment of death risk, as well as of predictors of such risk.
SMRs are calculated as the ratio of deaths observed in a cohort to those expected in a group of the same size from the general population in the same area and standardized for age and sex of the individuals in the study cohort. Current literature reporting mortality in rheumatic diseases varies widely, and therefore an accurate and weighted analysis of the SMR is of importance. The objective of this systematic review and meta-analysis of published data is to provide an accurate overview of the current risk of mortality in rheumatic diseases.
MethodsWe conducted a systematic review and meta-analysis by adapting the procedures of the Cochrane Collaboration (http://www.cochrane.org/training/cochrane-handbook) to a small local team. The findings are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement9 and MOOSE.10
Search strategySensitive electronic searches of MEDLINE (1950–June 2009), EMBASE (1980–June 2009), and Cochrane Library Plus were performed by trained researchers using comprehensive free text and MeSH synonyms of “mortality” and of “rheumatic diseases”, in general and by specific diagnoses (“rheumatoid arthritis”, “systemic lupus erythemathosus”, “systemic sclerosis”, “vasculitis”, “osteoarthritis”, “spondyloarthritis”, “dermatomyositis”, “osteoporosis”). We placed no restrictions on time or language of publication. For included articles, reference lists and the “Related articles” function on PubMed (www.pubmed.gov) were also assessed for possible inclusions. We also included abstracts of the ACR and EULAR meetings (2007–2008) and supplemented searches by checking references cited in published references.
Study selectionEligibility criteria were: (1) study population with rheumatoid arthritis, systemic lupus erythemathosus, systemic sclerosis, vasculitis, osteoarthritis, osteoporosis, dermatomyositis, or spondyloarthritis; (2) outcome of interest was mortality, and should be reported as an SMR, or this could be calculated; and (3) the accepted designs were cohorts or longitudinal observational studies of moderate to high quality. Studies on cancers, trauma, or infections related to the musculoskeletal system, as well as on congenital malformations, pregnancy or neonatal complications, or studies dealing solely with predictors of mortality but not reporting rates were excluded for this review.
Two authors independently assessed the records obtained by the electronic search results. They first screened titles and then abstracts in 10min sessions aided by EndNote®. If an article appeared related to the topic, the abstract was reviewed for eligibility. If there was any doubt, the full text of the article was retrieved and appraised for possible inclusion. Any differences among the two authors were discussed, and if necessary, a third author from the team was referred to for arbitration. A reason for exclusion was recorded in all cases if the article was not eligible or excluded.
Data abstraction and quality assessmentAssessment of risk of bias relied on the New Castle-Ottawa scale for cohorts,11 was appraised individually by two authors, the same whom independently extracted the data from included articles in forms previously pilot tested for feasibility and comprehensiveness. As this scale does not produce a definite score, we decided to assign a “high quality” to studies obtaining 7–8 stars, “moderate quality” if 5–6, and “low quality” any number of stars below these. Any study of low quality was excluded by protocol.
Data collected were the general characteristics of each study and the outcomes measured: study design, number of participants, rheumatic diagnosis and mean age at baseline of the patients and controls, reference population or sample, setting, calendar year, mean follow-up time, SMR (or risk ratio) and CIs, plus the raw observed and expected deaths (or deaths per group), total and by causes of mortality.
Statistical methodsOnly studies reporting SMR were included in the analysis. If an SMR or its 95% CI were not directly provided, then they were calculated from the reported observed (O) and expected (E) deaths, as SMR=OE and its 95% CI=SMR±1.96√O/E. Otherwise, the article was excluded. We calculated inverse variance weighted-pooled summary estimates of the SMRs (meta-SMRs). The meta-SMR represents a summary estimate of the increased risk of death in patients with rheumatic diseases compared with the general population, weighted by the inverse of the variance the log of the SMRs of each study. The meta-SMR was computed using a random-effects model and heterogeneity was tested for using the Q and I2 statistics. Calculations were performed on the log of the SMRs from the individual studies, and the resulting pooled values were then transformed back to the SMR scale. Separate meta-SMRs were calculated for specific diagnoses. Because heterogeneity is expected in meta-analyses of observational studies, meta-regression analysis was carried out to assess for sources of heterogeneity based on sample size, follow-up, age, percentage of women, comparator, quality, and country. All analyses were done using Stata statistical software, version 11 (StataCorp, College Station, TX, 2010).
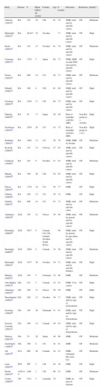
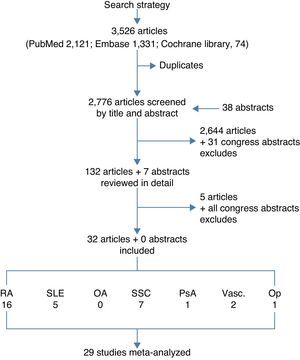
ResultsThe electronic searches yielded 3526 articles (2121 from PubMed, 1331 from EMBASE, and 74 from Cochrane Library), in all 2776 after removing duplicates, plus 38 abstracts from congresses. The selection by title and abstract left 132 articles plus 7 abstracts for detailed review. Only 32 studies met the complete set of selection criteria and were finally included in the review. Many studies were excluded for not providing the SMR or because the quality was not high enough. Fig. 1 shows the flow chart, and the list of all excluded studies and reasons are available upon request to the corresponding author. Table 1 shows the evidence table of all included studies, and Table 2 shows the results on mortality by study. The largest individual overall SMR came from studies on inflammatory diseases, and the specific SMR were very high for infections and pulmonary events.By disease, the populations included were: 16 studies on rheumatoid arthritis12–27 including data from around 10,000 patients (weighted mean age at baseline 54, and weighted mean follow-up 11 years); 5 studies on systemic lupus erythematosus28–32 that provided information on over 17,000 patients (mean weighted age at baseline 34, mean follow-up 14 years); 7 studies on systemic sclerosis33–39 accounting for over 1700 patients, mainly women aged 44 and followed up for an average of 17 years; a study in psoriatic arthritis40 including 680 patients (we found no studies on ankylosing spondylitis or other spondyloarthritis); 2 studies on Vasculitis41,42 accounting for over 345 patients (an additional article provided SMRs in Behçet's disease, but the display of SMRs was by age bands, not as a SMR for the general population); and one study on osteoporosis43 including 4311 patients. Only a study of osteoarthritis, a retrospective cohort, was reviewed in detail, although it finally did not meet the selection criteria.44 The study analyzed mortality in 296 women with osteoarthritis and a mean age of 57. Eighteen percent of the patients died, but the duration of the observation period was not specified, and the SMR not provided. The causes of mortality were very similar to those of the general population.
Evidence table of the included studies.
| Study | Disease | N | Mean follow-up (years) | Country | Age | % women | Outcomes | Reference | Qualitya |
| Alarcón (1995)12 | RA | 152 | 10 | UK | 61 | 70 | SMR, total and by specific causes | GP | Moderate |
| Björnadal (2002)13 | RA | 46,917 | 10 | Sweden | 71 | SMR, total and by specific causes | GP | High | |
| Book (2005)14 | RA | 152 | 12 | Sweden | 61 | 78 | SMR, total and by specific causes | GP | Moderate |
| Carmona (2007)15 | RA | 1578 | 5 | Spain | 60 | 72 | SMR, SMR on anti-TNF, total and by specific causes | GP | High |
| Doran (2002)16 | RA | 609 | 14 | US | 58 | 73 | SMR, total and by specific causes | GP | Moderate |
| Erhardt (1989)17 | RA | 108 | 8 | UK | 61 | 66 | SMR, total and by specific causes | GP | High |
| Goodson (2005)18 | RA | 1010 | 11 | UK | 60 | 72 | SMR, total and by specific causes | GP | High |
| Hakoda (2005)19 | RA | 91 | 17 | Japan | 56 | 80 | Risk of mortality with RA (HR) | Non-RA group (n=16,028) | High |
| Jacobsson (1993)20 | RA | 2979 | 20 | US | 43 | 75 | Risk of mortality with RA (RR) | Non-RA group (n=81) | High |
| Krishnan (2004)21 | RA | 3862 | 12 | US | 56 | 76 | SMR, SMR by decade | GP | Moderate |
| Kvalvik (2000)22 | RA | 147 | 15 | Norway | 57 | 65 | SMR, total and by specific causes | GP | High |
| Lindqvist (1999)23 | RA | 183 | 9 | Sweden | 51 | 63 | SMR, total and by specific causes | GP | Moderate |
| Minaur (2004)24 | RA | 100 | 40 | UK | 50 | 64 | SMR, total and by specific causes | GP | High |
| Pincus (2004)25 | RA | 1378 | 10 | US | 56 | 77 | SMR | GP | High |
| Gabriel (1999)26 | RA | 450 | 12 | US | 58 | 74 | SMR | GP | High |
| Gabriel (2003)27 | RA | 609 | 14 | US | 63 | 73 | SMR, total and by specific cause | GP | Moderate |
| Alamanos (2003)28 | SLE | 185 | 21 | Greece | 38 | 88 | SMR, total, by period, and by specific causes | GP | Moderate |
| Bernatsky (2006)29 | SLE | 9547 | 8 | Canada, US, UK, Iceland, Sweden, South Korea | 90 | SMR, total, by period, by age strata, and by specific causes | GP | High | |
| Bernatsky (2006)30 | SLE | 2688 | 9 | Canada | 34 | 90 | SMR, total and by specific causes | GP | Moderate |
| Björnadal (2004)31 | SLE | 4737 | 30 | Sweden | 59 | 71 | SMR, total and by specific features | GP | High |
| Manger (2002)32 | SLE | 338 | 5 | Germany | 32 | 86 | SMR | GP | Moderate |
| Abu-Shakra (1995)33 | SSc | 237 | 5 | Canada | 43 | 83 | SMR, % by causes | GP | High |
| Alamanos (2005)34 | SSc | 109 | 31 | Greece | 50 | 89 | SMR | GP | High |
| Hesselstrand (1998)35 | SSc | 249 | 13 | Sweden | 49 | 71 | SMR, total and by type of Scleroderma | GP | High |
| Jacobsen (1998)36 | SSc | 344 | 36 | Denmark | 41 | 81 | SMR, total and by type of Scleroderma | GP | High |
| Scussel-Lonzetti (2002)37 | SSc | 309 | 14 | Canada | 49 | 85 | SMR, total and by type of Scleroderma | GP | High |
| Simeon (2003)38 | SSc | 79 | 20 | Spain | 48 | 86 | SMR | GP | Moderate |
| Zarafonetis (1988)39 | SSc | 390 | 8 | US | 42 | 81 | SMR | GP | Moderate |
| Ali (2007)40 | PsA | 680 | 26 | Canada | 44 | 43 | SMR, estimated life-years lost | GP | Moderate |
| Lane (2005)41 | PSV | 99b | 2 | UK | 62 | 38 | SMR | GP | High |
| Booth (2003)42 | ANCA-V | 246c | 3 | UK | 66 | 43 | SMR | GP | Moderate |
| Center (1999)43 | OP | 4311 | 5 | Australia | 70 | SMR, by type of fracture | GP | High |
120 microscopic polyangiitis, 82 Wegener's granulomatosis, 33 renal limited vasculitis, 11 Churg-Strauss angiitis.
Abbreviations: RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; PsA, psoriatic arthritis; PSV, primary systemic vasculitis; ANCA-V, anca-associated vasculitis; OP, osteoporosis; SMR, standardized mortality ratio; LOS, longitudinal observational study; GP, general population.
Mortality in rheumatic diseases.
| Study | Disease | SMR | 95% CI | SMR women | SMR men | Other outcomes |
| Alarcón (1995)12 | RA | 1.95 | 1.28–2.83 | – | – | SMR by cause: CV 1.37, cancer 1.03, infection 11.31, respiratory 2.16 |
| Bjórnädal (2002)13 | RA | 2.03 | 2.00–2.05 | 2.09 (2.06–2.12) | 1.91 (1.87–1.95) | SMR by cause: CV 1.81, cancer 1.05, respiratory 2.82 |
| Book (2005)14 | RA | 1.56 | 1.28–1.88 | 1.61 (1.29–1.99) | 1.52 (0.99–2.23) | SMR by cause: CV 1.57, cancer 0.60, infection 3.08 |
| Carmona (2007)15 | RA | 1.49 | 1.17–1.87 | 1.46 (1.05–1.96) | 1.55 (1.06–2.19) | SMR by cause: CV 0.94, cancer 0.99, infection 18.68SMR if treated with anti-TNF: 0.52 (0.32–0.80) |
| Doran (2002)16 | RA | 1.27 | 1.13–1.41 | – | – | Causes of mortality: CV 37.4%, stroke 9.4%, lung disease 10%, cancer 10.3%, infection 15.2% |
| Erhardt (1989)17 | RA | – | – | – | – | SMR by cause: CV 3.00, cancer 1.80, infection 33.30, respiratory 5.00 |
| Goodson (2005)18 | RA | 1.70 | 1.46–1.98 | 1.84 (1.64–2.05) | 1.45 (1.22–1.71) | SMR by cause: CV 1.36, cancer 1.35, respiratory 1.79 |
| Hakoda (2005)19 | RA | 1.60a | 1.29–1.99 | 1.63 (1.26–2.06) | 1.40 (0.85–2.15) | SMR by cause: CV 1.30, cancer 0.98, respiratory 2.83 |
| Jacobsson (1993)20 | RA | 1.23b | 0.98–1.55 | – | – | SMR by cause: CV 1.77, cancer 1.50, respiratory 1.17 |
| Krishnan (2004)21 | RA | 1.28 | 0.94–1.75 | – | – | SMR if symptom onset before 1970: 1.60 (1.20–2.14) |
| SMR if symptom onset 1970–1979: 1.83 (1.45–2.30) | ||||||
| Kvalvik (2000)22 | RA | 1.49 | 1.15–1.88 | 1.68 (1.20–2.23) | 1.26 (0.81–1.81) | SMR by cause: CV 1.29, cancer 1.62 |
| Lindqvist (1999)23 | RA | 0.87 | 0.53–1.36 | – | – | Causes of mortality: CV 50%, Cancer 38%, Infections 5% |
| Minaur (2004)24 | RA | 1.15 | 1.04–1.27 | – | – | Survival was reduced by a median of 11 years for women and 10 years for men |
| Pincus (2004)25 | RA | 1.59 | 1.36–1.88 | – | – | |
| Gabriel (1999)26 | RA | 1.38 | 1.22–1.55 | 1.55 (1.34–1.78) | 1.07 (0.84–1.34) | Life reduced by 4 years in women and by 1 in men. |
| Gabriel (2003)27 | RA | 1.27 | 1.13–1.41 | 1.41 (1.22–1.61) | 1.08 (0.86–1.32) | |
| Alamanos (2003)28 | SLE | 1.50 | 1.30–1.80 | – | – | SMR (95% CI) 1981–1990: 1.60 (1.40–1.90)SMR (95% CI) 1991–2001: 1.50 (1.30–1.80) |
| Bernatsky (2006)29 | SLE | 2.40 | 2.30–2.50 | 2.50 (2.30–2.70) | 1.90 (1.70–2.20) | SMR by cause: vascular 1.7, cardiac 1.7, AMI 1.1, cancer 0.8, hematological cancer 2.1, lymphoma 2.8, lung cancer 2.3, infection 5.0, pneumonia 2.6, respiratory (other than pneumonia) 1.3, renal 7.9 |
| Bernatsky (2006)30 | SLE | 3.30 | 3.00–3.60 | – | – | SMR (95% CI) cerebrovascular cause 2.0 (1.0–3.7):Subaracnoid hemorrhage 1.4Other intracraneal hemorrhages 1.1Brain infarct 0.4Acute stroke 44.9Others 8.4 |
| Björnadal (2004)31 | SLE | 3.63 | 3.49–3.78 | – | – | SMR (95% CI) by features:Onset 20–39, 4.90Onset≥60, 2.911–3 flares, 3.24≥4 flares, 3.37Follow-up by rheumatology, 3.37Follow-up by others, 3.68 |
| Manger (2002)32 | SLE | 2.70 | – | – | – | 5-Year survival 96.6%Most common causes of death: CV (37%), infections (29%) |
| Abu-Shakra (1995)33 | SSc | 4.69 | 3.58–6.02 | 4.81 (3.65–6.44) | 4.18 (2.09–7.48) | The first cause was lung disease (29.5%), second renal and cardiac (both 11.5%) and others 28%. |
| Diffuse SSc | 6.18 | 4.17–8.81 | 5.60 (3.63–8.28) | 12.50 (4.06–29.17) | ||
| Limited SSc | 3.80 | 2.58–5.39 | 4.21 (2.73–6.22) | 2.69 (1.01–5.94) | ||
| Alamanos (2005)34 | SSc | 2.00 | 1.20–2.80 | 2.30 | 1.00 | 5-Year survival rate 83%10-Year survival rate 70% |
| Hesselstrand (1998)35 | SSc overall | 4.59 | 3.48–6.07 | 4.44 (2.87–6.34) | 4.77 (3.21–7.09) | 5-Year survival rate 86%10-Year survival rate 69% |
| Diffuse SSc | 6.06 | 4.09–9.02 | 5.22 (2.61–9.34) | 7.02 (3.74–12.00) | ||
| Limited SSc | 3.72 | 2.41–5.32 | 3.97 (2.17–6.66) | 3.46 (1.72–6.18) | ||
| Jacobsen (1998)36 | SSc overall | 2.90 | 2.50–3.40 | 2.70 (2.30–3.30) | 3.70 (2.70–5.10) | |
| Diffuse SSc | 4.50 | 3.50–5.70 | – | – | ||
| Limited SSc | 2.30 | 1.80–2.80 | – | – | ||
| Scussel-Lonzetti (2002)37 | SSc | 2.69 | 2.10–3.40 | 2.55 (1.90–3.3) | 1.76 (0.80–3.30) | Causes of death (%): systemic sclerosis 53, cancer 19.7, atherosclerosis 15.1, other causes 12.1 |
| Diffuse SSc | 6.17 | 2.80–11.70 | 8.06 (3.20–16.50) | 5.83 (0.70–21.00) | ||
| Limited SSc | 2.71 | 1.85–3.80 | 2.84 (1.90–4.00) | 1.10 (0.20–3.20) | ||
| Simeon (2003)38 | SSc | 4.29 | 2.22–7.50 | – | – | 5-Year survival 71%10-Year survival 64%15-Year survival 62% |
| Zarafonetis (1988)39 | SSc | 5.40 | 3.58–8.65c | 5.00 (2.04–14.69) | 5.60 (3.46–9.52) | |
| Ali (2007)40 | PsA | 1.36 | 1.12–1.64 | 1.47 (1.13–1.91) | 1.25 (0.95–1.65) | Estimated life-years lost: 2.99 years (95% CI 1.14, 4.77) |
| Lane (2005)41 | PSV | 4.80 | 2.90–6.60 | 3.05 (1.20–4.90) | 5.90 (3.10–8.80) | |
| Booth (2003)42 | ANCA-V | 2.83 | 2.24–3.60 | – | – | 1-Year survival 84%5-Year survival 76% |
| Center (1999)43 | OP+hip fx | – | – | 2.18 (2.03–2.32) | 3.17 (2.90–3.44) | |
| OP+vertebral fx | – | – | 1.66 (1.51–1.80) | 2.38 (2.17–2.59) | ||
| OP+major fx | – | – | 1.92 (1.70–2.14) | 2.22 (1.91–2.52) | ||
| OP+minor fx | – | – | 0.75 (0.66–0.84) | 1.45 (1.25–1.65) |
CI not provided by authors, obtained from total number of deaths 142/390 and expected deaths 26.28/390.
Abbreviations: RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSc, systemic sclerosis; PsA, psoriatic arthritis; PSV, primary systemic vasculitis; ANCA-V, anca-associated vasculitis; OP, osteoporosis; fx, fracture; SMR, standardized mortality ratio.
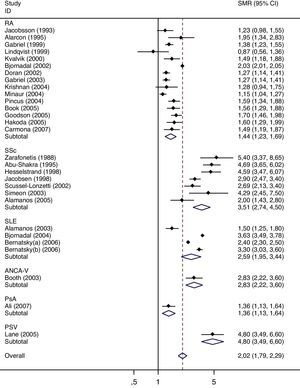
The results of the meta-analysis are shown in Table 3. The overall pooled SMR in the studied diseases is 2.03 (95% CI 1.79–2.29), ranging from 1.36 in psoriatic arthritis to 4.80 in vasculitis. Figs. 2 and 3 show the forest plots. Heterogeneity was large; however, the analysis of such heterogeneity within diseases (rheumatoid arthritis, lupus, and systemic sclerosis) did not provide any hint on the source, as no association was found with any of the studied: sample size, follow-up, age, percentage of women, comparator (general population versus non-rheumatoid), quality (high versus moderate), and country (US & Canada versus Europe and Others).
Results of the meta-analysis of mortality, by disease and sex, and overall.
| Total | Women | Men | |||||||||||||
| 95% CI | 95% CI | 95% CI | |||||||||||||
| n | SMR | Low | upp | I2 (%) | n | SMR | Low | upp | I2 (%) | n | SMR | Low | upp | I2 (%) | |
| RA | 15 | 1.44 | 1.23 | 1.69 | 96.4 | 8 | 1.66 | 1.44 | 1.92 | 90.3 | 8 | 1.39 | 1.11 | 1.73 | 90.5 |
| SSc | 7 | 3.51 | 2.74 | 4.50 | 81.2 | 5 | 3.50 | 2.60 | 4.72 | 76.6 | 5 | 3.86 | 2.81 | 5.31 | 56.0 |
| SLE | 4 | 2.59 | 1.95 | 3.44 | 98.8 | 1 | 2.50 | 2.32 | 2.70 | 1 | 1.90 | 1.64 | 2.20 | ||
| ANCA-V | 1 | 2.83 | 2.23 | 3.60 | |||||||||||
| PsA | 1 | 1.36 | 1.13 | 1.64 | 1 | 1.47 | 1.13 | 1.91 | 1 | 1.25 | 0.95 | 1.65 | |||
| PSV | 1 | 4.80 | 3.49 | 6.60 | 1 | 3.05 | 1.90 | 4.90 | 1 | 5.90 | 3.96 | 8.80 | |||
| OPa | 1 | 2.18 | 2.05 | 2.32 | 1 | 3.17 | 2.92 | 3.44 | |||||||
| Overall | 29 | 2.03 | 1.79 | 2.29 | 98.2 | 17 | 2.08 | 1.89 | 2.29 | 90.4 | 17 | 2.07 | 1.72 | 2.49 | 95.0 |
Results show the meta standardized mortality ratio (SMR) with lower (low) and upper (upp) limits of the 95% confidence interval (CI), and the I2 statistic showing heterogeneity.
We have performed a systematic review and meta-analysis on the standardized mortality in rheumatic diseases. The major result is that mortality is generally increased, especially in inflammatory conditions, and it is supported by moderate to high quality cohort studies. The causes of death in our review actually match those of the general population, where cardiovascular events and cancer are the most frequent causes, but notably, infections and respiratory complications of the underlying inflammatory diseases are actually the causes related to an excess mortality in the diseases of interest.
It draws our attention that many mortality studies do not report an SMR neither an expected rate, despite drawing conclusions on mortality rates in individual diseases apparently higher than expected. In some cases, instead of an SMR, the authors reported survival curves with the disease versus expected survival curves, thus providing graphic evidence but no numbers.
Another finding of this comparative study is that the two diseases in which mortality has been studied in higher depth and with better quality studies are rheumatoid arthritis and systemic sclerosis. Two meta-analyses have been published previously on the issue of mortality in rheumatoid arthritis51,52; however, they were centered in cardiovascular mortality, not reporting data on overall mortality. In both studies, cardiovascular mortality was higher than expected, although not clearly in the inception cohorts included, indirectly supporting an aggressive early approach in rheumatoid arthritis to control inflammation and therefore controlling cardiovascular inflammation. Ioannidis et al. published an outstanding meta-analysis on mortality in systemic sclerosis of patient reported data from several scleroderma cohorts.45 Unfortunately, a pooled estimate was not deemed adequate given the heterogeneity of SMRs. The main cause of death was cardiovascular disease, followed by pulmonary disease, or mostly both. Less frequent causes were cancer, renal crisis, and infection. After the review was finished, Wolfe et al. published a mortality study in fibromyalgia,46 with an overall SMR of 0.90 (95% CI 0.61–1.26), thus not demonstrating an increased death risk in this condition. Our review shows important gaps in the knowledge of mortality in osteoarthritis and spondyloarthritis.
Regarding meta-analysis of mortality of other diseases, Canavan et al. published a meta-analysis on the mortality of Crohn's disease, reporting a pooled SMR of 1.52 (95% CI 1.32–1.74),47 and Jess et al. one in ulcerative colitis, with SMRs varying from 0.7 to 1.4.48 A pooled analysis in HIV reported SMRs of 1.30 (95% CI 1.06–1.58) among homosexual men and 9.37 (95% CI 8.13–10.75) in drug abusers,49 and another one in Cushing's disease showed an SMR of 2.2 (1.45–3.41).50 Therefore our findings may be described as worse than other inflammatory diseases, such as bowel diseases, and even worse than HIV, except for HIV in drug abusers.
Our study has several limitations, the main one being the broad scope of the population. By studying many diseases at the same time, we are gaining perspective on the specialty, but loosing precision and detailed information. An analysis by disease would have been more adequate to understand predictive factors, as well as causes of death and other variables related to severity.
An adequate risk assessment of patients during follow-up must include an assessment of death risk, as well as of predictors of such risk.6,7 Nowadays, it is possible to develop automatic systems that capture specific signals from patients’ data and then alert the physician; such systems are invaluable to improve safety and quality of care, as they increase physician's awareness of a worse prognosis, or of specific risks, such as allergies, interactions, or others, without increasing consultation times.8 As a first step towards developing such an IT solution, our group deemed important to feed the system with data on frequent comorbidities, risk of death, and most frequent causes of death in the rheumatic diseases. In order to apply our results, we will introduce algorithms in our computer system, by which situations such as an admission due to a respiratory condition, or to an infectious disease, will prompt the system to increase awareness about the individual patient, based on risk and priorities.
Based on our results and on the good quality of the included studies, we can conclude that rheumatic diseases increase in general the risk of death, and especially inflammatory diseases.
ConclusionsThe major result is that mortality is generally increased, especially in inflammatory conditions, and it is supported by moderate to high quality cohort studies. The causes of death in our review actually match those of the general population, where cardiovascular events and cancer are the most frequent causes, but notably, infections and respiratory complications of the underlying inflammatory diseases are actually the causes related to an excess mortality in the diseases of interest.
Based on our results and on the good quality of the included studies, we can conclude that rheumatic diseases increase in general the risk of death, and especially inflammatory diseases.
Ethical disclosuresProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Funding sourcesThis work was partially supported by the RETICS Program, RD08/0075 (RIER), from Instituto de Salud Carlos III (ISCIII), within the VI Plan Nacional de I+D+I 2008–2011 (FEDER).
Conflicts of interestAll authors declare no conflicts of interest.
We would like to thank Research Unit of Sociedad Española de Reumatología (Madrid, Spain) for the collaboration in this manuscript.