In Mexico, other risk factors are associated with hepatitis C virus (HCV): prior heroin users, living alone, widower, and northern region residence. Rheumatoid arthritis (RA) patients are considered immunosuppressed and HCV testing is recommended before treatment. The aim of the study was to describe the characteristics of HCV testing in RA patients in three different medical care settings in a non-endemic area.
MethodsA retrospective observational study was performed using medical records from 960 RA patients describing the indications for HCV testing.
ResultsThe test was performed in 28.6% and the HCV overall frequency was 0.36%. Population characteristics were not associated with an increased risk of HCV infection; therefore, anti-HCV positivity was low. The main reason for testing was before starting biological agents.
ConclusionDue to the low pre-test probability, testing for HCV infection should be personalized; i.e., according to disease prevalence in a particular geographical location and the individual risk factors.
En México, se han descrito factores de riesgo para virus de hepatitis C (VHC), además de los conocidos como: consumo de heroína, individuos que viven solos, ser viudo y residencia en el norte del país. Los pacientes con artritis reumatoide (AR) son considerados inmunodeprimidos y se recomienda realizar pruebas de VHC antes del inicio del tratamiento. El objetivo fue describir las características de pacientes con AR a quienes se realizaron pruebas de VHC.
Material y métodosEstudio observacional, retrospectivo de 960 registros médicos donde se describieron las indicaciones para las prueba de VHC.
ResultadosLa prueba se realizó en el 28.6% y la frecuencia global de VHC fue de 0.36%. Las características de la población no se asociaron con un mayor riesgo de infección, por lo tanto la presencia de anti-VHC fue baja. La principal razón para realizar la prueba fue el inicio de tratamiento biológico.
ConclusiónDebido a la baja probabilidad pre-test, las pruebas para el VHC deben ser personalizadas, es decir, según la prevalencia de la enfermedad de acuerdo a la zona geográfica y los factores de riesgo individuales.
The global prevalence of hepatitis C virus (HCV) is 2.35%, and 160 million individuals are chronically infected.1 Seroprevalence of HCV antibodies in Mexican population is 1.4% (95% CI 1.1–1.6%); this is equivalent to 700,000 infected adults (95% CI 568,000–830,000).2 The risk factors for HCV infection are injection drug use, reception of blood products prior to 1992, reception of clotting factor concentrates before 1987, long-term hemodialysis, needle-stick injuries among healthcare workers, and patient-to-patient transmission resulting from poor infection control practices. Other risk factors include having been born to an HCV-infected mother, having been incarcerated, and having received a tattoo in an unregulated setting.3 Risk factors for HCV infection in Mexican population in addition to those mentioned are prior heroin users [OR=9.8, 95% CI (2.1–41.4)], living alone [OR=2.6, 95% CI (1.1–5.9)], being a widower [OR=2.2, 95% CI (1.1–4.3)] and northern region residence [OR=1.9, 95% CI (1.1–3.2)].2 HCV testing is recommended in select populations based on demographics, prior exposure, high-risk behaviors, and medical conditions.3
The risk of infection in RA depends in three different situations: RA itself as a chronic disorder with immunological dysfunctions, immunocompromising comorbidities, and the use of potent immunomodulatory drugs.4 International5 and Mexican rheumatoid arthritis (RA) guidelines recommend testing for hepatitis B and C in high-risk patients (level of evidence: 4; grade of recommendation: D) before the use of non-biologic and biologic disease-modifying anti-rheumatic drugs (DMARDs).6 Although patients with a higher risk profile for HCV have been defined by the Mexican National Health Survey2 and the Centers for Disease Control and Prevention, RA patients are not in this epidemiological group. Therefore, implementation of the recommendations for HCV testing in this population has not been clearly accomplished. The aim of the study is to describe the characteristics of HCV testing in patients with RA in three different medical care settings in a non-endemic area.
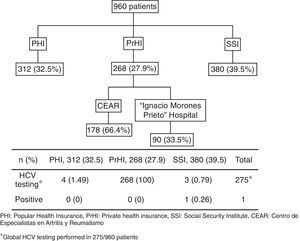
MethodsWe performed a retrospective study based on clinical medical records. RA patients who fulfilled the American College of Rheumatology (ACR) 1987 and/or the ACR/European League Against Rheumatism (EULAR) 2010 classification criteria were included.7,8 The patients were from three different health systems from Mexico: Popular Health Insurance at the UANL University Hospital, PHI; the Mexican Social Security Institute for private sector workers (Instituto Mexicano del Seguro Social, IMSS), SSI; and Private health insurance at the Arthritis and Rheumatism Specialist Center (Centro de Especialistas en Artritis y Reumatismo CEAR) and Hospital Central “Dr. Ignacio Morones Prieto”, PrHI.
We describe the indications for HCV testing and its overall prevalence. This study was approved by the local Institutional Review Board with registration no. RE-13010.
We performed a descriptive analysis using the following variables: age, gender, time since RA diagnosis, treatment, risk factors for HCV, hepatitis B virus (HBV) and HIV, including: tattoo, marital status, prior blood transfusion and prior illicit drug. Also, we evaluated liver function tests and HCV result by ELISA. In addition, we determined the main reasons for HCV testing. Results were reported as mean and standard deviation for continuous variables and percentages for categorical variables.
ResultsA total of 960 patients were evaluated; 312 (32.5%) were from PHI, 380 (39.5%) from SSI, and 268 (27.9%) from the PrHI. Eight hundred eighty-five (92%) and mean age was 51.6 years (SD 12.99). The mean time since RA diagnosis to testing was 9.8 years (SD 8.2). Rheumatoid factor was positive in 554 (67.6%) of 819 patients and anti-cyclic citrullinated peptide (anti-CCP) antibody in 239 (69.8%) of 342 patients. Two hundred and seventy-three (28.4%) patients were using biologic agents for RA treatment and the rest were on synthetic DMARDs. The mean value for transaminases was normal. Alanine transaminase (ALT) had a mean of 29IU/L (SD 12.8) and aspartate transaminase (AST) a mean of 25IU/L (SD 10.7).
The HCV testing was performed in 28.6% (275/960). HCV testing was as follows: PHI 4/312 patients (1.49%), SSI 3/380 (0.79%) patients, and PrHI 268/268 (100%) patients (Fig. 1). The main reasons for HCV testing at PHI and SSI are detailed in Table 1. The only reason for requesting HCV testing at PrHI was prior to the beginning of biological therapy. The overall frequency of HCV was 0.36% (1/275).
HCV risk factors in PHI and SSI patients and main reason for screening.
| Patient | Age | Marital status | Prior blood transfusion | Prior illicit drugs | Tattoo | Main reason for HCV screening |
|---|---|---|---|---|---|---|
| 1 | 33 | Married | – | – | – | aLFT |
| 2 | 44 | Married | Yes | – | – | DD |
| 3 | 45 | Married | – | – | – | aLFT |
| 4 | 57 | Married | – | – | – | aLFT |
| 5 | 39 | Married | – | – | – | Prior biologic |
| 6 | 62 | Married | – | – | – | Prior biologic |
| 7 | 62 | Separated | Yes | – | – | DD |
268 patients belong to PrHI were screening prior use of biologic treatment.
HCV, hepatitis C virus; PHI, public health insurance; SSI, Social Security Institute; PrHI, private health insurance; aLFT, abnormal liver function test; DD, differential diagnosis.
We found that only 28.6% of patients were screened for HCV and this depended on the medical care setting, and not necessarily represent the adherence to clinical practice guidelines. It is well known that the main reasons for requesting HCV testing are the following: before starting biological agents, as a differential diagnosis in patients with acute polyarthritis, the presence abnormal liver function tests, and/or belonging to HCV high risk groups (previous use of illicit drugs, transfusions before 1965, etc.).
In the PrHI, the main reason for HCV testing was the starting of biological agents for RA treatment (surpassing the other risk factors), as stated in international recommendations.5
In PHI and SSI we observed different reasons for testing, such as differential diagnosis, abnormal liver function tests, and the beginning of biologic treatment. Since most patients already had confirmed and established RA, additional testing for differential diagnosis without HCV risk factors was not made.
Previously, Valdespino et al. described the risk factors associated with a high HCV prevalence in Mexico. The area where the study was conducted is associated with a higher prevalence compared to other areas of the country [OR 1.9, 95% CI (1.1–3.2)]. The age [50–59 years-old, OR 1.3, 95% CI (0.8–2.1)] and gender [OR 1.1, 95% CI (0.8–1.5)] of our patients were not associated with an increased risk of HCV infection according to prior study.2
We emphasized that HCV infection frequency is low in our RA patients living in northern Mexico. We observed that the only risk factor in our patients was the geographical location, although other risk factors as: living alone and widower was not assessed.
The Global Burden of Diseases, Injuries, and Risk Factors 2010 (GBD2010) is an international collaborative effort to estimate the burden of disease using available parameters. Anti-HCV seroprevalence was estimated by GBD as high (>3.5%), moderate (1.5–3.5%), and low (<1.5%).9 Although anti-HCV positivity in this cohort was 0.36%, which is considered low, it could not be clarified with this study design.
This study represents three main different medical settings (PHI, SSI and PrHI) for a global view in Mexican population. The Mexican health system comprises two sectors, public and private. Within the public sector are social security institutions: IMSS, Institute for Social Security and ISSSTE, Social Security Services for Civil Servants, Petroleos Mexicanos, the Ministry of Defense, the Secretary of the Navy, and others. Institutions and programs serving the population without social security are the Health Secretariat, State Health Services, the IMSS-Oportunidades Program and PHI. The private sector includes insurance companies and service providers in offices, clinics and private hospitals, and providers of alternative medicine.10
The low pre-test probability in this population with a low prevalence of HCV justifies testing personalization. This is the opposite of published by Conway et al. that evaluated 100 medical records of RA patients and retrospectively analyzed testing for hepatitis B virus and HCV. They recommended testing for HCV even in non-endemic population.11 We differ from the Conway statement because we believe that medicine should be predictive, personalized, preventive, and participatory (P4 medicine).12 P4 medicine includes “personalized” medicine because it is based on genetic information of each individual. It should be “predictive” because this personalized information will determine the risk of certain diseases in each individual. It should be “preventive” because by predicting a certain risk, prophylactic measures may be established to decrease it and finally, it is “participatory” because many of these prophylactic interventions require patient participation.13 P4 medicine may reduce disease and therefore improve well-being.14
The reasons for not doing HCV testing in the other patients were not evaluated. Maybe, the patient characteristics analyzed in this study (as female gender and age), are not considered high risk factors for HCV infection based on local epidemiology.2
According to the frequency of HIV infection, the risk, including the administration of anti-TNF drugs in a patient with chronic infection, appears to be low. Previously, Brunasso et al. studied the safety of the administration of anti-TNF drugs in patients with chronic HCV infection and inflammatory diseases, such as RA. They found that of 153 cases, only 2 patients worsened under this therapy.15 Actually, it is reported that the anti-TNF drugs may participate in the pathogenesis of HCV reducing inflammation by inhibiting TNF.16
One of the limitations of the study is that we did not evaluate a priori other risk factors of the population studied. Although the study was made in three different medical settings which represent a 64.1% of the medical insurance in our country, we did not include other medical institutions. In the case of IMSS the approbation of the use of biologics agents in RA patients, now has been changed and all the patients even if they not have risk factors for HCV is necessary perform the screening. RA patients from a non-endemic HCV population are screened for HCV in a 28.6%, probably because particularities of the population. For patients from PrHI, we did not evaluate other factors (other than the indication of the beginning of biological drugs) that may contribute to HCV screening application.
We conclude that a cost-benefit testing HCV and other viruses (HIV, HBV) should be according to the personalization of the population, i.e., the prevalence of the disease in a particular geographical location, the individual risk factors, and the risk of the drug serious side-effects (HCV activation).
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflicts of interest or financial disclosures.
CEAR group: Diana Flores-Alvarado, Jacqueline Rodríguez-Amado, Lorena Pérez-Barbosa, Jannet Riega-Torres, Dionicio Galarza-Delgado, Miguel Villarreal-Alarcón.