To explore the association between T. gondii and autoimmune rheumatic diseases (ARDs).
MethodsThis study involved 82 patients with ARDs: 44 rheumatoid arthritis (RA), 28 systemic lupus erythematosus (SLE), and 10 systemic sclerosis (SSc) and 61 age- and sex-matched controls. Sociodemographic, clinical, and laboratory data were collected, and disease activity was assessed. Exposure to toxoplasmosis risk factors was investigated. Serological tests for anti-Toxoplasma IgM and IgG antibodies were assessed using ELISA.
ResultsIn SLE patients, a significant difference of T. gondii IgM versus controls was detected (P=.03). In RA and SLE patients, T. gondii IgG showed a significant difference versus controls (34 (77.3%) P=.001 and 18 (64.3%) P=.03, respectively). There was no significant difference in SSc versus controls. Fetal congenital anomalies displayed a significant difference in IgM seropositive compared to seronegative patients (P=.04). Cat exposure showed a significant difference between IgM and IgG seropositive versus seronegative patients (12 (80.0%) P=.02 and 34 (59.6) P=.04, respectively). There was no significant difference in seropositive patients regarding history of abortion, neuro-psychiatric manifestations, disease activity parameters (ESR, CRP), or different regimens of medications.
ConclusionToxoplasma IgM seropositivity is associated with SLE patients. T. gondii IgG seropositivity is associated with both RA and SLE patients. However, Toxoplasma seropositivity had no association with SSc patients. An association between fetal congenital anomalies and IgM seropositivity was demonstrated. A linkage between cat exposure as a risk factor and toxoplasmosis was suggested among ARD patiants. Exploration of impact of toxoplasmosis on ARDs is a necessity through randomized controlled trials.
Explorar la asociación entre Toxoplasma gondii y enfermedades reumáticas autoinmunes (ERA).
MétodosEste estudio involucró a 82 pacientes con ERA: 44 con artritis reumatoide (AR), 28 con lupus eritematoso sistémico (LES) y 10 con esclerosis sistémica (SSc); y 61 controles emparejados por edad y sexo. Se recopilaron datos sociodemográficos, clínicos y de laboratorio, y se evaluó la actividad de la enfermedad. Se indagó exposición a factores de riesgo de toxoplasmosis. Las pruebas serológicas de anticuerpos IgM e IgG antitoxoplasma se evaluaron mediante ELISA.
ResultadosEn pacientes con LES se detectó una diferencia significativa de T. gondii IgM vs. controles (p = 0,03). En pacientes con AR y LES, T. gondii IgG mostró una diferencia significativa frente a los controles (34 [77,3%] p = 0,001 y 18 [64,3%] p = 0,03, respectivamente). No hay diferencia significativa en SSc vs. controles. Las anomalías congénitas fetales mostraron una diferencia significativa en los pacientes seropositivos para IgM en comparación con los pacientes seronegativos (p = 0,04). La exposición a los gatos mostró una diferencia significativa entre los pacientes seropositivos para IgM e IgG frente a los seronegativos (12 [80%] p = 0,02 y 34 [59,6] p = 0,04, respectivamente). No hubo diferencias significativas en pacientes seropositivos con respecto a antecedentes de aborto, manifestaciones neuropsiquiátricas, parámetros de actividad de la enfermedad (ESR, CRP) o diferentes regímenes de medicamentos.
ConclusiónLa seropositividad a toxoplasma IgM se asocia con pacientes con LES. La seropositividad de IgG frente a T. gondii se asocia tanto con pacientes con AR como con LES. Sin embargo, la seropositividad a toxoplasma no tuvo asociación con pacientes con SSc. Se demostró una asociación entre anomalías congénitas fetales y seropositividad a IgM. Se sugirió un vínculo entre la exposición a los gatos como factor de riesgo y la toxoplasmosis entre los pacientes con ERA. La exploración del impacto de la toxoplasmosis en las ERA es una necesidad a través de ensayos controlados aleatorios.
Autoimmune rheumatic diseases (ARDs) are multisystem chronic diseases associated with a high rate of morbidity and mortality in both developed and developing countries. ARDs include a wide disease spectrum: rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and systemic sclerosis (SSc). RA involves chronic synovial joint inflammation, which may proceed to severe disability.1,2 SLE exhibits a relapsing and remittent clinical course with very heterogeneous clinical picture, associated with multi-organ involvement.3 On the other hand, SSc is characterized by excessive fibrosis, vascular injuries, and immune disturbances.4 Geo-epidemiological studies implied that host genetic susceptibility and environmental factors, including socioeconomic status, dietary habits, environmental pollutants, ultraviolet radiation exposure, and infections act as potential risks or protective factors in the susceptibility to ARDs.5
Toxoplasma gondii (T. gondii) is an obligate intracellular protozoan that is claimed to be associated with autoimmunity. In both developed and developing countries, one-third of the populations are infected with T. gondii.6 It has a complex life cycle where cats and other felines represent the definitive host. The oocysts excreted in cat stool spread to the environment and cause infection of a very wide range of mammals and birds. During the acute stage, the parasite can invade almost all types of nucleated cells and multiplies rapidly as tachyzoites, while in the chronic stage, it targets the brain and the muscles and multiplies as bradyzoites inside true tissue cysts. Toxoplasmosis outcome is related to the immune status in humans: in immunocompetent individuals, T. gondii infection is usually asymptomatic and is mostly followed by a latent infection.7 Latent toxoplasmosis refers to the chronic form of asymptomatic toxoplasmosis which constitutes most cases, while serious disease occurs in infants and immunocompromised patients. In addition, reactivation of latent toxoplasmosis frequently occurs in immunocompromised patients and results in uncontrolled proliferation of the parasite.8
Infection may contribute to the development of ARDs through molecular mimicry and epitope spreading. While molecular mimicry includes cross-reaction between the host tissue and the pathogen, epitope spreading results in activation of antigen-presenting cells by the pathogen leading to an enhanced local presentation of self-antigens that causes T cell activation.9 Prandota and colleagues10 reviewed the association between late chronic toxoplasmosis and ARDs and highlighted the role of iron, folic acid, and iodine deficiencies that result from toxoplasmosis in the establishment of ARDs.
Increased prevalence of ARDs, resultant disability, and increased medical costs cause more economic burden, which requires more understanding of the pathophysiology of these diseases for better control. Therefore, we tried to explore the prevalence of anti-Toxoplasma antibodies of both IgM and IgG classes in patients with ARDs, including RA, SLE, and SSc and evaluate the association of ARDs with toxoplasmosis-related risk factors
Patients and methodsStudy designThis case-control study was carried out on 82 patients with ARDs (44 RA, 28 SLE, and 10 SSc). These patients were recruited from those attending the Rheumatology and Immunology Unit (inpatient and outpatient), Mansoura University Hospital, from January 2019 to February 2020. The inclusion criteria included: (a) age≥18 years (b) classified as having one of ARDs as follows: RA was diagnosed according to the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) criteria for the diagnosis of RA,11 SLE was diagnosed as stated by the International Collaborating Clinics (SLICC) criteria,12 while SSc was diagnosed according to the 2013 ACR/EULAR classification criteria.13 Those with diabetes mellitus, hepatitis C virus, or overlap with other connective tissue diseases were excluded from the study. Sixty-one age- and sex-matched healthy controls were also enrolled in the study.
Patients’ demographic, clinical and therapeutic dataFor the included ARD patients, sex, age, pregnancy outcomes, socioeconomic status, occupation, residence, and associated comorbidities were reported. All patients were asked about any previous or current significant neuro-psychiatric insults. Therapeutic history of immunosuppressive medications was also documented including corticosteroids, mycophenolate mofetil (MMF), conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), and biological disease-modifying antirheumatic drugs (bDMARD). All patients gave history of receiving csDMARDs.
Disease activity scoresAll ARD patients were evaluated by a well-trained rheumatologist. The evaluation included a comprehensive physical examination with targeted systemic evaluation. The disease activity was assessed in all patients as follows; RA patients were evaluated using Disease Activity Score-28 joints (DAS-28),14 and SLE patients were evaluated by using SLE disease activity index 2000 (SLEDAI-2K),15 while SSc patients were assessed with modified Rodnan skin score (mRSS).16
Toxoplasmosis risk factorsAll participants were asked to complete a self-administered questionnaire to evaluate risk factors of toxoplasmosis acquisition. It was designed to be easily filled. Patients of low education were guided to fill in the questionnaire. The questionnaire included the following items: chronic exposure to cats, kittens, or cat feces, ingestion of unpasteurized cow's and goat's milk, and consumption of unwashed raw vegetables.
Sampling and laboratory testsEight ml venous blood was withdrawn from each patient on the same day of clinical evaluation. Serum samples were obtained from centrifuged blood samples were stored at −20°C until analysis. Then, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were assessed by latex agglutination tests (Leaner Chemicals Co., Spain). Serological tests for anti-T. gondii IgM and IgG were assessed using ELISA kits (Catalog#: TX022G, Calbiotech, Inc., USA), following the manufacturer's instructions.
Statistical analysisData were analyzed using the Statistical Package of Social Science (SPSS) program for Windows (Standard version 24). The normality of data was first tested with one-sample Kolmogorov–Smirnov test. Qualitative data were defined using number and percentage. Association between categorical variables was tested using Chi-square test while Fischer exact test was used when expected cell count less than 5. Continuous variables were displayed as mean±SD (standard deviation) for normally distributed data and median (min–max) for non-normal data. The two groups were compared with Student t test for normal data and Mann–Whitney test for non-normal data. Significant variables entered Logistic regression model using the forward wald statistical technique to predict the most significant elements and to control for possible interactions and confounding effects. The results were considered significant when P≤0.05.
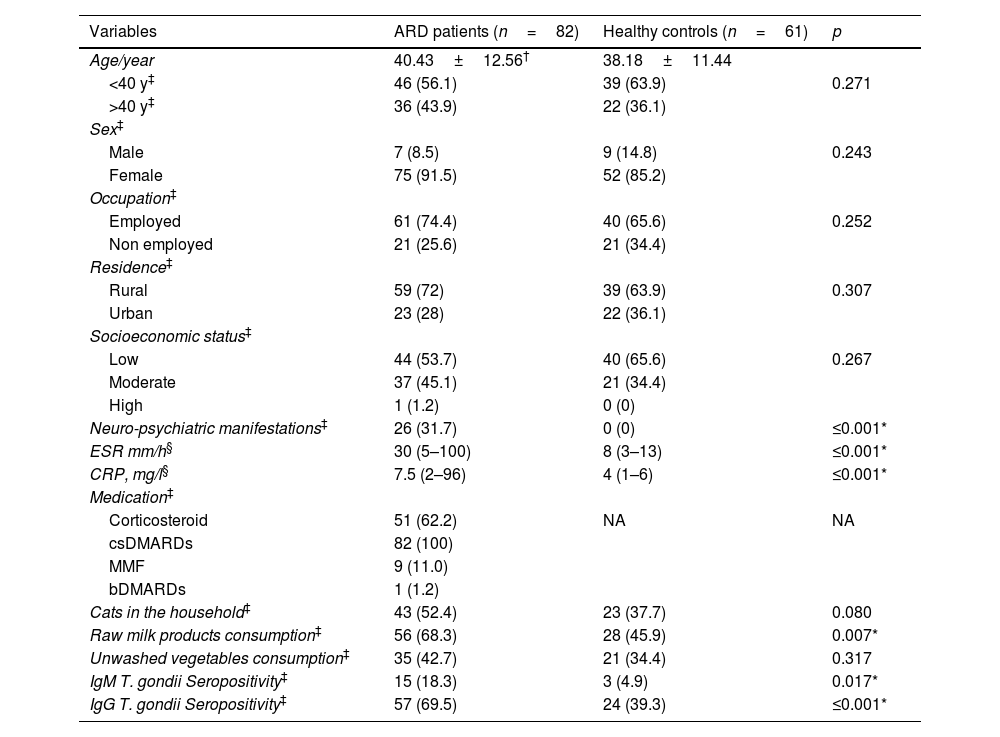
ResultsThis study included 82 ARD patients: 44 (53.75%) RA, 28 (34.1%) SLE, and 10 (12.2%) SSc. The mean age was 40.43±12.56 years, and 75 patients (91.5%) were females. Neuro-psychiatric manifestations were reported in 26 (31.7%) ARD patients, significantly higher than healthy controls (P≤0.001). Anti-T. gondii IgM was detected in 15 (18.3%) ARD patients and in 3 (4.9%) controls (P=0.017). Anti-T. gondii IgG antibodies were also detected in 57 (69.5%) patients and 24 (39.3%) healthy controls (P≤0.001). Noteworthy, both IgM and IgG antibodies were detected in 13 patients (15.9%) and in only 3 (4.9%) controls. Raw milk and milk products consumption showed a significant proportion in ARD patients (68.3%) compared to the controls (45.9%) (P=0.007), as shown in Table 1.
Sociodemographic data, clinical, and laboratory characteristics, therapeutic regimens, Toxoplasma related risk factors, and T. gondii seroprevalence among ARD patients (n=82) and healthy controls (n=61).
| Variables | ARD patients (n=82) | Healthy controls (n=61) | p |
|---|---|---|---|
| Age/year | 40.43±12.56† | 38.18±11.44 | |
| <40 y‡ | 46 (56.1) | 39 (63.9) | 0.271 |
| >40 y‡ | 36 (43.9) | 22 (36.1) | |
| Sex‡ | |||
| Male | 7 (8.5) | 9 (14.8) | 0.243 |
| Female | 75 (91.5) | 52 (85.2) | |
| Occupation‡ | |||
| Employed | 61 (74.4) | 40 (65.6) | 0.252 |
| Non employed | 21 (25.6) | 21 (34.4) | |
| Residence‡ | |||
| Rural | 59 (72) | 39 (63.9) | 0.307 |
| Urban | 23 (28) | 22 (36.1) | |
| Socioeconomic status‡ | |||
| Low | 44 (53.7) | 40 (65.6) | 0.267 |
| Moderate | 37 (45.1) | 21 (34.4) | |
| High | 1 (1.2) | 0 (0) | |
| Neuro-psychiatric manifestations‡ | 26 (31.7) | 0 (0) | ≤0.001* |
| ESR mm/h§ | 30 (5–100) | 8 (3–13) | ≤0.001* |
| CRP, mg/l§ | 7.5 (2–96) | 4 (1–6) | ≤0.001* |
| Medication‡ | |||
| Corticosteroid | 51 (62.2) | NA | NA |
| csDMARDs | 82 (100) | ||
| MMF | 9 (11.0) | ||
| bDMARDs | 1 (1.2) | ||
| Cats in the household‡ | 43 (52.4) | 23 (37.7) | 0.080 |
| Raw milk products consumption‡ | 56 (68.3) | 28 (45.9) | 0.007* |
| Unwashed vegetables consumption‡ | 35 (42.7) | 21 (34.4) | 0.317 |
| IgM T. gondii Seropositivity‡ | 15 (18.3) | 3 (4.9) | 0.017* |
| IgG T. gondii Seropositivity‡ | 57 (69.5) | 24 (39.3) | ≤0.001* |
Median (Min–Max), P value by student t-test, Mann–Whitney test, Chi-square test, Fisher's exact test, Monte carlo test.
ARD: autoimmune rheumatic disease, CRP: C reactive protein, ESR: erythrocyte sedimentation rate, MMF: mycophenolate mofetil, csDMARDs: conventional synthetic disease-modifying antirheumatic drugs, bDMARDs: biological disease-modifying antirheumatic drugs, NA: not applicable, IgM: immunoglobulin M, IgG: immunoglobulin G.
Using DAS28 scoring system, 18 RA patients showed moderate activity, 10 with mild activity, and 9 with severe activity. Using SLEDAI scoring system, there were 12 SLE patients with moderate activity, 10 with severe activity and only 6 with mild activity. Using MRSS scoring system, 6 SSc patients showed moderate activity and 4 displayed mild activity (data not shown). There was non-significant difference regarding the relationship between IgM and IgG seropositivity of T. gondii and the disease activity either in RA, SLE, or SSc (IgM: P=0.37, P=0.81, P=0.74 and IgG: P=0.80, P=0.37, P=0.43, respectively).
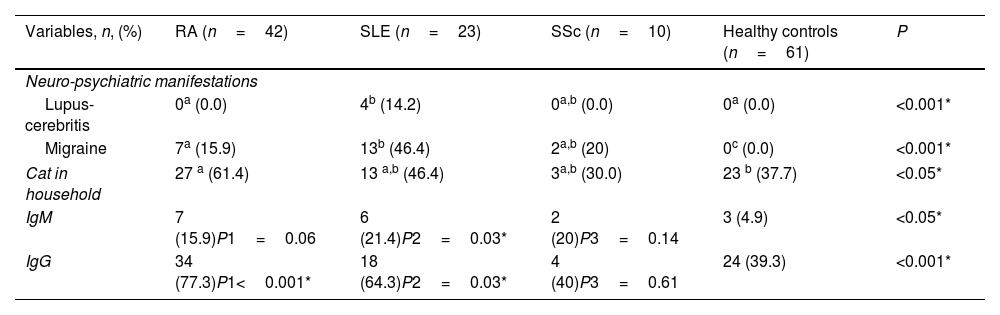
Patients with ARDs were assessed based on their autoimmune disease. T. gondii IgM was detected in 6 (21.4%) patients with SLE, 7 (15.9%) patients with RA, and only 2 (20%) with SSc, nevertheless, significant difference was reported in SLE patients versus control group (P=0.03). Contrastingly, T. gondii IgG was detected in 34 (77.3%) RA, 18 (64.3%) SLE, and 4 (40%) SSc patients, with significant difference in both RA and SLE patients versus the control group (P<0.001, P=0.03, respectively). Neuro-psychiatric manifestations were reported in 17 (60.7%) SLE patients: 4 (14.2%) cases with lupus-cerebritis and 13 (46.4%) patients with migraine with highly significant difference in comparison to RA and controls (P<0.001). In SSc patients, migraine showed significant difference compared to the controls (P<0.001). Cats’ contact with RA patients showed significant difference versus control group (Pv<0.05), as shown in Table 2.
Neuropsychiatric manifestations and T. gondii seroprevalence among ARD patients (n=82) and healthy controls (n=61).
| Variables, n, (%) | RA (n=42) | SLE (n=23) | SSc (n=10) | Healthy controls (n=61) | P |
|---|---|---|---|---|---|
| Neuro-psychiatric manifestations | |||||
| Lupus-cerebritis | 0a (0.0) | 4b (14.2) | 0a,b (0.0) | 0a (0.0) | <0.001* |
| Migraine | 7a (15.9) | 13b (46.4) | 2a,b (20) | 0c (0.0) | <0.001* |
| Cat in household | 27 a (61.4) | 13 a,b (46.4) | 3a,b (30.0) | 23 b (37.7) | <0.05* |
| IgM | 7 (15.9)P1=0.06 | 6 (21.4)P2=0.03* | 2 (20)P3=0.14 | 3 (4.9) | <0.05* |
| IgG | 34 (77.3)P1<0.001* | 18 (64.3)P2=0.03* | 4 (40)P3=0.61 | 24 (39.3) | <0.001* |
RA: rheumatoid arthritis, SLE: systemic lupus erythromatosis, SSc: systemic sclerosis, IgM: immunoglobulin M, IgG: immunoglobulin G.
Significant P≤0.05. Data are presented as numbers and percentages. P value by Kruskal–Wallis test and compact letters demonstrate pairwise comparison (similar letters=statistically insignificant difference, different letters=statistically significant difference). P1: RA versus healthy controls, P2: SLE versus healthy controls, P3: SSc versus healthy controls.
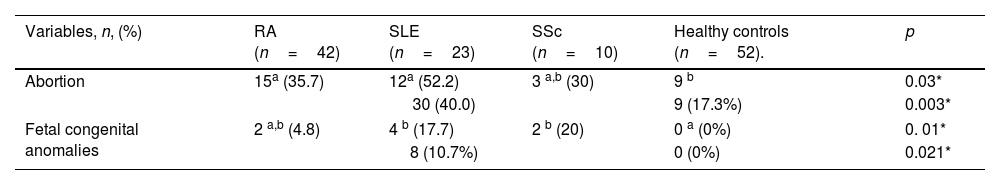
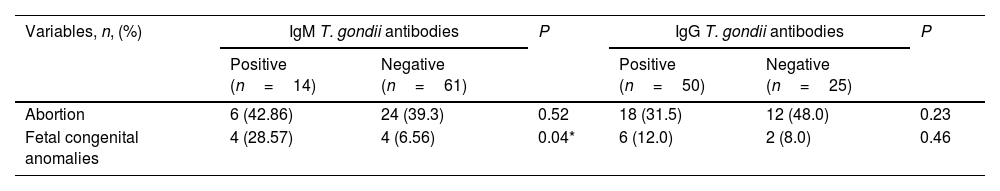
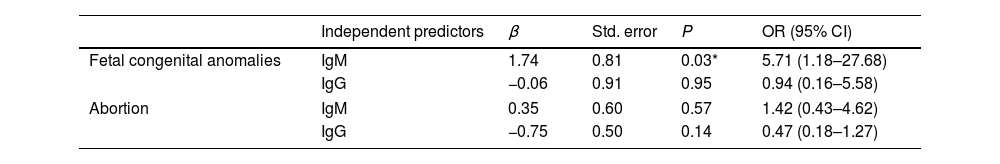
Abortion was reported in 30 ARD patients (40%, P=0.003). There was a significant difference between ARDs patients (SLE and RA) and the control group regarding history of abortion (P=0.03). First trimester abortions were reported in most cases, followed by second trimester abortions. Fetal congenital anomalies, mostly cardiovascular malformations, were reported in 8 (10.7% P=0.021) female ARD patients’ offspring with a significant difference in SLE and SSc in comparison to the control group (P=0.01), as illustrated in Table 3. Abortion displayed non-significant difference in IgM seropositive patients. However, fetal congenital anomalies showed significant difference in IgM seropositive patients (P=0.04), compared to IgM seronegative patients (Table 4). In addition, toxoplasmosis seropositive IgM antibodies were a robust predictor of prenatal congenital abnormalities with a statistically significant difference (P=0.03), as illustrated in Table 5. There were non-significant differences between IgG seropositive and seronegative groups in terms of fetal congenital abnormalities or history of abortion (Tables 4 and 5).
Pregnancy outcomes among female ARD patients (n=75) and healthy controls (n=52).
| Variables, n, (%) | RA (n=42) | SLE (n=23) | SSc (n=10) | Healthy controls (n=52). | p |
|---|---|---|---|---|---|
| Abortion | 15a (35.7) | 12a (52.2) | 3 a,b (30) | 9 b | 0.03* |
| 30 (40.0) | 9 (17.3%) | 0.003* | |||
| Fetal congenital anomalies | 2 a,b (4.8) | 4 b (17.7) | 2 b (20) | 0 a (0%) | 0. 01* |
| 8 (10.7%) | 0 (0%) | 0.021* | |||
ARD: autoimmune rheumatic disease.
Association between T. gondii seropositivity and pregnancy outcomes among female ARD patients (n=75).
| Variables, n, (%) | IgM T. gondii antibodies | P | IgG T. gondii antibodies | P | ||
|---|---|---|---|---|---|---|
| Positive (n=14) | Negative (n=61) | Positive (n=50) | Negative (n=25) | |||
| Abortion | 6 (42.86) | 24 (39.3) | 0.52 | 18 (31.5) | 12 (48.0) | 0.23 |
| Fetal congenital anomalies | 4 (28.57) | 4 (6.56) | 0.04* | 6 (12.0) | 2 (8.0) | 0.46 |
P value by Chi-square test, and Fisher's exact test. IgM: immunoglobulin M, IgG: immunoglobulin G.
Logistic regression analysis for abortion and fetal congenital anomalies and T. gondii antibodies in female ARDs patients.
| Independent predictors | β | Std. error | P | OR (95% CI) | |
|---|---|---|---|---|---|
| Fetal congenital anomalies | IgM | 1.74 | 0.81 | 0.03* | 5.71 (1.18–27.68) |
| IgG | −0.06 | 0.91 | 0.95 | 0.94 (0.16–5.58) | |
| Abortion | IgM | 0.35 | 0.60 | 0.57 | 1.42 (0.43–4.62) |
| IgG | −0.75 | 0.50 | 0.14 | 0.47 (0.18–1.27) | |
IgM: immunoglobulin M, IgG: immunoglobulin G, P value by Logistic regression, OR: odds ratio, CI: confidence interval, std error: standard error.
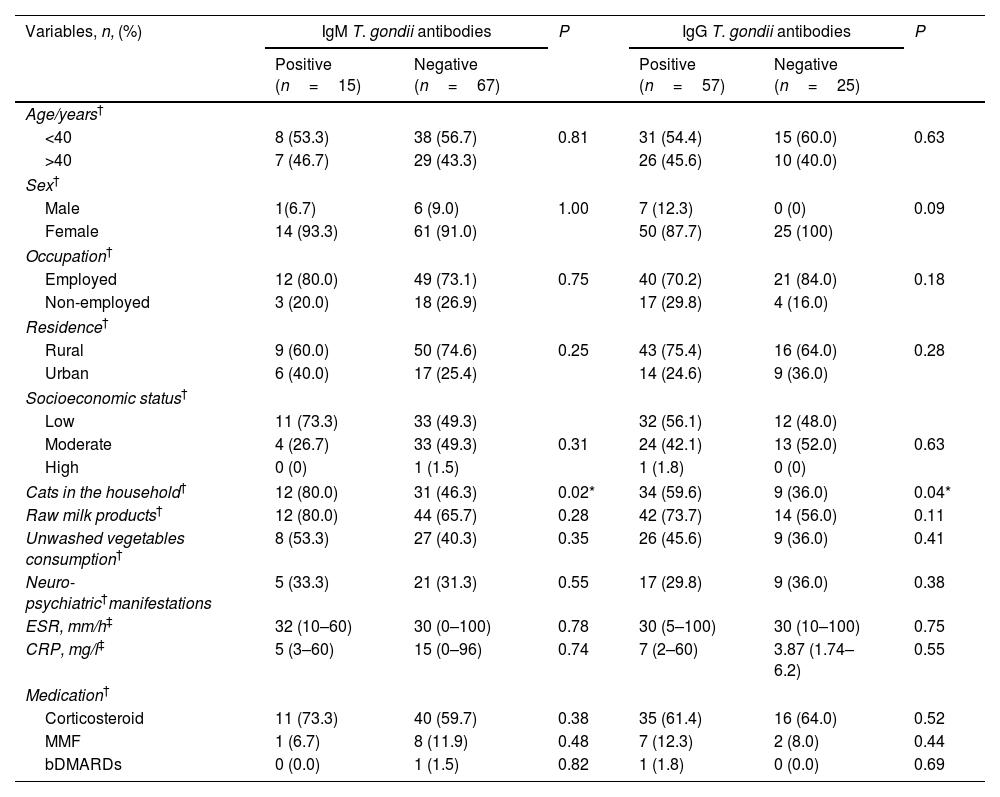
Interestingly, neither neuro-psychiatric manifestations nor disease activity parameters (ESR and CRP) showed any significant differences regarding Toxoplasma seropositivity. There was a significant difference between IgM and IgG Toxoplasma seropositivity and cats in the household (12 (80.0%) versus 12 (46.3%), P=0.02 and 34 (59.6) versus 9 (36.0%), P=0.04, respectively), as illustrated in Table 6.
The linkage between T. gondii seroprevalence and demographic variables, related risk factors, clinical and laboratory data, and therapeutic regimens among ARD patients.
| Variables, n, (%) | IgM T. gondii antibodies | P | IgG T. gondii antibodies | P | ||
|---|---|---|---|---|---|---|
| Positive (n=15) | Negative (n=67) | Positive (n=57) | Negative (n=25) | |||
| Age/years† | ||||||
| <40 | 8 (53.3) | 38 (56.7) | 0.81 | 31 (54.4) | 15 (60.0) | 0.63 |
| >40 | 7 (46.7) | 29 (43.3) | 26 (45.6) | 10 (40.0) | ||
| Sex† | ||||||
| Male | 1(6.7) | 6 (9.0) | 1.00 | 7 (12.3) | 0 (0) | 0.09 |
| Female | 14 (93.3) | 61 (91.0) | 50 (87.7) | 25 (100) | ||
| Occupation† | ||||||
| Employed | 12 (80.0) | 49 (73.1) | 0.75 | 40 (70.2) | 21 (84.0) | 0.18 |
| Non-employed | 3 (20.0) | 18 (26.9) | 17 (29.8) | 4 (16.0) | ||
| Residence† | ||||||
| Rural | 9 (60.0) | 50 (74.6) | 0.25 | 43 (75.4) | 16 (64.0) | 0.28 |
| Urban | 6 (40.0) | 17 (25.4) | 14 (24.6) | 9 (36.0) | ||
| Socioeconomic status† | ||||||
| Low | 11 (73.3) | 33 (49.3) | 32 (56.1) | 12 (48.0) | ||
| Moderate | 4 (26.7) | 33 (49.3) | 0.31 | 24 (42.1) | 13 (52.0) | 0.63 |
| High | 0 (0) | 1 (1.5) | 1 (1.8) | 0 (0) | ||
| Cats in the household† | 12 (80.0) | 31 (46.3) | 0.02* | 34 (59.6) | 9 (36.0) | 0.04* |
| Raw milk products† | 12 (80.0) | 44 (65.7) | 0.28 | 42 (73.7) | 14 (56.0) | 0.11 |
| Unwashed vegetables consumption† | 8 (53.3) | 27 (40.3) | 0.35 | 26 (45.6) | 9 (36.0) | 0.41 |
| Neuro-psychiatric†manifestations | 5 (33.3) | 21 (31.3) | 0.55 | 17 (29.8) | 9 (36.0) | 0.38 |
| ESR, mm/h‡ | 32 (10–60) | 30 (0–100) | 0.78 | 30 (5–100) | 30 (10–100) | 0.75 |
| CRP, mg/l‡ | 5 (3–60) | 15 (0–96) | 0.74 | 7 (2–60) | 3.87 (1.74–6.2) | 0.55 |
| Medication† | ||||||
| Corticosteroid | 11 (73.3) | 40 (59.7) | 0.38 | 35 (61.4) | 16 (64.0) | 0.52 |
| MMF | 1 (6.7) | 8 (11.9) | 0.48 | 7 (12.3) | 2 (8.0) | 0.44 |
| bDMARDs | 0 (0.0) | 1 (1.5) | 0.82 | 1 (1.8) | 0 (0.0) | 0.69 |
The relationship between Toxoplasma infection and ARDs has been investigated in different studies with debatable results; anti Toxoplasma IgG seropositivity has shown an association with RA and SSc in European patients; however, Latin American patients lacked this relationship.17 Contrastingly, other studies remarked the protective effect exerted by parasitic infections against ARDs.18
In this study, both anti-Toxoplasma IgM and IgG antibodies in ARD patients were found to be higher than healthy controls (18.3%, 69.5%, and P=0.017, P≤0.001, respectively), supported by the study done by other studies.19,20 Here, the highest prevalence of anti-Toxoplasma IgG antibodies was reported in RA followed by SLE, then SSc patients (79.5%, 64.3%, 40.0%, and P=0.038 respectively), consistent with Fischer and colleagues21 who documented a higher prevalence of anti-Toxoplasma IgG in RA compared to SLE patients. IgM Toxoplasma seropositivity among SLE patients was revealed to have significant difference in comparison to the control group, consistent with the study done by Wilcox, and colleagues.22 Also, a significant difference of IgG Toxoplasma seropositivity was demonstrated in both RA and SLE patients versus the control group. In agreement with that, an association of IgG Toxoplasma seropositivity among RA and SLE was reported regarding latent toxoplasmosis.23 Of note, 13 patients and 3 controls were found to have both types of antibodies. While such cases are commonly described as having reactivation of chronic toxoplasmosis, Dhakal and colleagues24 concluded that IgM may persist in the patients’ sera for years, hence, chronic cases may be misdiagnosed as having reactivation. Therefore, confirmation of an acute or chronic infection should depend on testing at a reference laboratory.
There was no correlation between T. gondii IgM and IgG seropositivity and disease activity parameters in terms of ESR and CRP in any of the studied diseases. In agreement with these findings, some studies reported no correlation between Toxoplasma seropositivity and ESR or CRP.21 Latent toxoplasmosis with its chronic effects and ARDs with its fluctuating course, necessitate cohort studies on large scales.
Although toxoplasmosis is considered a potential risk factor for ARDs, the specific mechanism of induction or exacerbation is not fully understood. Altered T cell repertoire was reported in RA,25 and dysregulation of cellular and humoral immune responses was documented in SLE patients as well.26 On the other hand, toxoplasmosis may induce the progression of chronic diseases. Toxoplasmosis promotes IL-17 expression, which contributes to the pathophysiology of several ARDs. Toxoplasma was proved to act as a ligand for Toll-like receptors (TLR); thus, chronic activation of these receptors favors the production of autoantibodies.27 Mimics between SLE activity and manifestations of infection have been observed in many patients causing difficult management of the disease.
Neuro-psychiatric manifestations including lupus-cerebritis and migraine showed significant difference between SLE and controls. SLE is characterized by a wide range of neuro-psychiatric manifestations.28 In RA, neurological affection is multifactorial whether local joint changes, extra-articular rheumatoid nodules, or secondary vasculitis are included. In this study, migraine in SSc and RA patients showed significant difference versus controls. There is an issue of discussion about neuro-psychiatric manifestations in SSc. Noteworthy, some psychiatric disorders like mania and impaired cognitive functions in patients with bipolar disorder were linked to the concentration of Toxoplasma IgM in patients’ sera.29 In agreement with Voss and Stangel,30 neuro-psychiatric manifestations, particularly lupus-cerebritis and migraine were more prevalent in SLE patients. It was reported that bipolar disorder, obsessive compulsive disorder, attention deficit hyperactivity disorder, anti-social personality disorder, drug abuse, generalized anxiety and panic disorder, autism, schizophrenia, mood disturbances, homicide, and suicide occur more frequently in Toxoplasma infected patients than in normal controls.31 Unfortunately, the development of neuro-toxoplasmosis in SLE patients was found to be usually misdiagnosed as neuro-psychiatric SLE.23 In this study, Neuro-psychiatric manifestations showed non-significant difference in relation to Toxoplasma seropositivity. Many studies were done to explore the role of T. gondii as a potential factor for development of neuro-psychiatric manifestation using serological methods, however debate is still found. Much information is lacking concerning molecular characteristic, pathophysiological, and neurobiological mechanisms of toxoplasmosis.
Among female ARD patients, a significant association between anti-T. gondii IgM seropositivity and history of offspring's fetal congenital anomalies was demonstrated, consistent with a study done on aborted women showed a significant association between toxoplasmosis and RA.32 Children born to mothers suffering from SLE may have neonatal lupus syndrome, including congenital anomalies with a high risk of congenital heart diseases.33 There was non-significant association between T. gondii seropositivity and abortion. A variable range of pregnancy outcomes and subsequent sequelae after birth, including abortion, stillbirth, hydrocephalus, chorioretinitis, cerebral calcification, mental retardation, and learning difficulties, was associated with toxoplasmosis. In addition, a correlation between ARDs with abortion and intrauterine fetal deaths was documented.34 Pregnancy outcome complications were documented in RA patients with disease activity; however, there are debates concerning pregnancy outcomes in SSc patients.
A significant correlation was found between anti-T. gondii IgM, IgG antibodies and cats’ exposure in ARD patients. This factor elucidates the role played by cats as a source of T. gondii transmission in ARD patients. In agreement with these results, Tian et al.35 detected an association between contact with cats and toxoplasmosis prevalence, as well as a significant correlation between Toxoplasma seropositivity and arthritis in China. Neverthless, some studies reported no association between anti-T. gondii antibodies and contact with cat.36 Awareness about the role played by cats in acquisition of toxoplasmosis is important. More efforts are needed to control toxoplasmosis in cat. Also, more accurate studies on larger sample size are valuable.
Interestingly, SSc patients showed no significant link to Toxoplasma seropositivity. Little is known about the link between SSc and Toxoplasma, however, the immune response against chronic toxoplasmosis favoring T helper type (Th) 2 over Th1 response substantiates the hygiene hypothesis, which considers toxoplasmosis and other infections as protective factors against ARDs.37 Conversely, Arnson et al.38 found a positive correlation between SSc and Toxoplasma seroprevalence and concluded that Toxoplasma might play a role in triggering SSc. Molecular mimicry, super-antigen, and endothelial damage were proposed to explain how infections act as triggering cofactors in the immuno-pathogenesis of SSc.
In this study, no significant association was exhibited in T. gondii seropositive ARD patients who received different regimens of medications. The combined pyrimethamine and azithromycin with corticosteroids is recommended regimen for treating ocular toxoplasmosis in immunocompromised patients although there is a leakage in clinical trials.39 Nevertheless, in a murine model of latent toxoplasmosis using ME-49 strain, more T. gondii cysts in the brain and encephalitis were detected when long-term corticosteroid therapy was applied.40 An increased risk of opportunistic infections among treated patients with immunosuppressive drugs as bDMARDs was documented and probably may lead to life-threatening toxoplasmosis.41 The risk of reactivation of latent toxoplasmosis in patients receiving immunosuppressive medications promotes question. A limitation of this study is that the sample size is small with only one patient received bDMARDs. Also, the study is of cross-sectional nature, however, it is not fully granted that randomized controlled trial could be done to assess the impact of toxoplasmosis on ARDs. Also, because of active disease and/or the existence of antiphospholipid antibodies, SLE patients have more abortions than healthy controls, these aspects were not fulfilled.
ConclusionToxoplasmosis has shown a diverse relationship with ARDs in different studies. This study highlighted the association between IgM Toxoplasma seropositivity and SLE, and between T. gondii IgG seropositivity and both RA and SLE. Nevertheless, Toxoplasma seropositivity had no association with SSc patients. A significant association was demonstrated between anti-T. gondii IgM seropositivity and history of offspring's fetal congenital anomalies. Besides, cats’ exposure in ARD patients might be a risk factor in toxoplasmosis. Randomized controlled trial with large number of patients is a necessity to explore the actual impact of toxoplasmosis on ARDs whether hazardous or protective.
Authors’ contributionsWafaa A. Aboukamar involved in conceptualization, methodology, software, writing manuscript, data curation, and reviewing manuscript. Samar Habib involved in conceptualization, writing manuscript, and reviewing manuscript. Samar Tharwat and Mohamed Kamal Nassar involved in conceptualization, collection of data, and writing manuscript. Manal A. Elzoheiry involved in laboratory investigation and revising manuscript. Rania Atef involved in collection of data. Manar S. Elmehankar involved in laboratory investigation, reviewing manuscript.
Ethical approvalThe study was approved by Mansoura Faculty of Medicine-Institutional Review Board (MFM-IRB) with a code number of R/20.11.1095.
ConsentThe study was explained to all participants, and written informed consent was signed by all of them.
Data availability statementData would be made available on reasonable request.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare no conflict of interest.
All patients and healthy controls who participated in this study are appreciated. We would like to thank Dr. Adnan Elmasry and Dr. Daniela Isaacs for the Spanish translation.