Behçet's disease (BD) is a systemic inflammatory disease with various presentations. The data on the course of BD in Egyptian patients are limited.
ObjectivesThe objective of the study was to describe the evolution and association of the different phenotypes of BD.
Material and methodsThis chronological cohort study included adult Egyptian patients suffering from BD. Demographic data and the chronological order of the disease's manifestations were collected.
ResultsThe study included 233 patients. Their mean age at the onset of the disease was 26.3±6.9 years. The mean duration from onset of the disease to meeting the criteria was 11.2±30.3 months. The mean duration of the disease was 96.8±72.2 months. On onset of the disease, the most common phenotypes were mucocutaneous (84.5%), musculoskeletal (15.9%), ocular (14.6%) and peripheral venous disease (PVD) (7.3%); on the other hand, pulmonary, peripheral arterial and great vessel phenotypes evolved several years after onset of the disease. The mean time from meeting the criteria to the evolution of a new phenotype was 53.8±58.7 months. Associations between the different phenotypes were observed: PVD and superficial thrombophlebitis, peripheral arterial disease and PVD; another association was also observed between aortic involvement and cerebrovascular disease.
ConclusionBD could continue to evolve several years after onset of the disease, making the previous belief about BD yield questionable. BD tends to respect the anatomy of the affected system. Some phenotypes tend to coexist, suggesting a shared aethiopathogeny and that the disease is of a systemic nature.
La enfermedad de Behçet (BD) es una enfermedad inflamatoria sistémica con diversas presentaciones. Los datos sobre el curso de la BD en pacientes egipcios son limitados.
ObjetivosEl objetivo del estudio fue describir la evolución y la asociación de los diferentes fenotipos de BD.
Material y métodosEste estudio de cohorte cronológico incluyó pacientes egipcios adultos que sufren de BD. Se recopilaron datos demográficos y el orden cronológico de las manifestaciones de la enfermedad.
ResultadosEl estudio incluyó a 233 pacientes. Su edad media al inicio de la enfermedad fue de 26,3±6,9 años. La duración media desde el inicio de la enfermedad hasta el cumplimiento de los criterios fue de 11,2±30,3 meses. La duración media de la enfermedad fue de 96,8±72,2 meses. Al inicio de la enfermedad, los fenotipos más comunes fueron los fenotipos mucocutáneos (84,5%), musculoesqueléticos (15,9%), oculares (14,6%) y la enfermedad venosa periférica (7,3%); por otro lado, la enfermedad pulmonar, arterial periférica y fenotipos de grandes vasos evolucionaron varios años después del inicio de la enfermedad. La duración media desde el cumplimiento de los criterios hasta la evolución de un nuevo fenotipo fue de 53,8±58,7 meses. Se observaron asociaciones entre los diferentes fenotipos: enfermedad venosa periférica y tromboflebitis superficial, enfermedad arterial periférica y enfermedad venosa periférica; también se observó otra asociación entre la afectación aórtica y la enfermedad cerebrovascular.
ConclusiónLa BD podría continuar evolucionando varios años después del inicio de la enfermedad, haciendo cuestionable la previa creencia del rendido BD. La BD tiende a respetar la anatomía del sistema afectado. Algunos fenotipos tienden a coexistir, lo que sugiere una etiopatogenia compartida y una naturaleza sistemática de la enfermedad.
Behçet's disease (BD) is a chronic relapsing inflammatory disease. Its highest prevalence is seen along the Silk Road. Due to the protean disease manifestations, different clinical phenotypes exist including the mucocutaneous, musculoskeletal, ocular, neurological, cardiac, vascular and gastrointestinal phenotypes. Few studies have addressed the chronological pattern of BD evolution among specific ethnicities.1–4 Most of the studies addressing the associations between the different phenotypes were conducted in specialty units and were concerned with a specific phenotype.5–7 The heterogenic nature of the disease demographics and clinical characteristics, including the disease onset, the chronological order of development of the protean manifestations and the associations between the different phenotypes, has been observed even within the same ethnic group. Identification of the chronological pattern of disease development could enable the anticipation of future organ involvement and the development of strategies for patient screening and follow-up. Moreover, the recognition of the associations and combinations of the different phenotypes will guide physicians towards the appropriate investigations to detect the subclinical involvement of possibly affected organs.1–3 This study aimed to describe the BD phenotypes in a cohort of Egyptian patients: their evolution and associations.
Material and methodsThis was a chronologic retrospective cohort study involving 233 adult BD patients included in the Egyptian vasculitis cohort8 and following at the Rheumatology and Rehabilitation Department and Outpatient Clinic at Kasr Al-Ainy Hospital, Cairo University, between 2010 and 2017. All patients fulfilled the 2006 International Criteria for BD.9
We analyzed the data collected from the patients’ records including the demographic features and chronologic pattern of development of the different phenotypes. Disease duration, follow-up period, duration from the disease onset to criteria fulfilment and the duration from diagnosis to the evolution of a new clinical phenotype were reported. Disease onset was defined as the onset of the first disease manifestation; whereas disease duration was calculated from the time of criteria fulfilment to the time of the last visit. Regarding the duration from the disease onset to criteria fulfilment, zero refers to the disease onset. Concerning the duration between manifestation development and criteria fulfilment, and the duration of phenotype shift, zero represents the time of criteria fulfilment while negative values refer to the development of the manifestations prior to criteria fulfilment and positive values refer to the development of the manifestations following criteria fulfilment.
Twelve clinical phenotypes were considered: the mucocutaneous, musculoskeletal, ocular, neurological, peripheral venous, peripheral arterial, pulmonary, cardiac, aortic, vena caval, renal and gastrointestinal phenotypes. A certain organ system was considered to be affected upon involvement of its parenchymal and/or vascular structures. The following items were recorded for each phenotype: the cumulative frequency, being an onset manifestation and the time of its development in relation to the time of criteria fulfilment.
The study conformed to the provisions of the Declaration of Helsinki and was approved by the local Research and Ethics Committee of the Rheumatology and Rehabilitation Department of Kasr Al-Ainy Hospital.
Statistical analysisCategorical variables are described in terms of frequency and percentage; while numerical variables are described in terms of mean±standard deviation (SD). Statistical differences between groups were tested using the chi-square tests with the calculation of the odds ratio (OR) and the 95% confidence interval (95% CI). A two-tailed probability value (P value) less than 0.05 was considered statistically significant. All statistical calculations were performed using SPSS (Statistical Package for the Social Science; SPSS, Inc., Chicago, IL, United States of America) version 15 for Microsoft Windows.
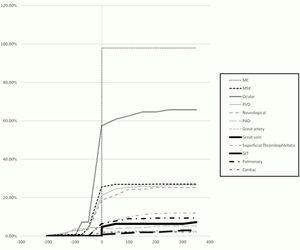
ResultsThe study population included 233 patients. The demographic characteristics of the patients are demonstrated in Table 1. The chronological order of involvement of the different organ systems is presented in Table 2. Among the mucocutaneous manifestations as the most common onset features, oral ulcers were the most prevalent (78.6%) followed by genital ulcers (64.3%). The cumulative frequency of the appearance of the different clinical phenotypes in relation to the time of criteria fulfilment is shown in Fig. 1.
The demographic characteristics of Behçet's disease patients.
| N=233 | Range | Mean±SD |
|---|---|---|
| Age of onset (year) | 10–49 | 26.3±6.9 |
| Follow-up period (month) | 0–336 | 29.7±45.6 |
| Disease duration (month) | 0–384 | 96.8±72.2 |
| Age at criteria fulfilment (year) | 16–49 | 27.2±7.1 |
| Duration from onset to criteria fulfilment (month)a | 0–204 | 11.2±30.3 |
| Duration of shift from one phenotype to another after criteria fulfilment (month)b | 1–348 | 53.8±58.7 |
The chronological order of involvement of the different organ systems in Behçet's disease patients.
| N=233 | Cumulative frequencyN (%) | Being the onset manifestationN (%) | Duration between manifestation development and criteria fulfilment (month)a | |
|---|---|---|---|---|
| Range | Mean±SD | |||
| Mucocutaneous | 228 (97.9%) | 197 (84.5) | −204 to 0 | −8.4±27.8 |
| Arthritis | 63 (27%) | 37 (15.9) | −168 to 180 | 6.7±51.8 |
| Ocular | 153 (65.7%) | 34 (14.6) | −74 to 240 | 18.2±42.9 |
| Neurological | 59 (25.3%) | 11 (4.7) | −60 to 156 | 30.9±46 |
| Cardiac | 5 (2.1%) | 1 (0.4) | −24 to 38 | 11.8±26 |
| Pulmonary | 22 (9.4%) | 2 (0.9) | −24 to 180 | 44.5±54.4 |
| PVD | 62 (26.6%) | 17 (7.3) | −96 to 276 | 27.5±60.9 |
| PAD | 12 (5.2%) | 0 (0) | 0 to 172 | 47.3±63.9 |
| Aortic disease | 6 (2.6%) | 0 (0) | 0 to 105 | 69.5±39.2 |
| Vena caval disease | 17 (7.3%) | 3 (1.3) | 0 to 348 | 51.4±82.6 |
| GIT | 7 (3%) | 0 (0) | 1 to 228 | 101.2±87.8 |
PVD: peripheral venous disease, PAD: peripheral arterial disease, GIT: gastrointestinal.
Disease onset coincided with criteria fulfilment in 139 (59.7%) patients. A mean duration of 11.2±30.3 months elapsed between the first disease manifestation and criteria fulfilment. Of the 233 patients, 158 (67.8%) patients developed a new clinical phenotype during the disease course with a mean duration of 53.8±58.7 months from the time of criteria fulfilment to the evolution of a new phenotype: the new phenotype developed within the first three years after criteria fulfilment in 91 (57.6%) patients, three to five years after criteria fulfilment in 17 (10.8%) patients, and more than five years after criteria fulfilment in 50 (31.6%) patients. Sixty five patients had mild disease that was limited to the cutaneous and musculoskeletal systems during the first three years after diagnosis; among those patients, 42 (64.6%) patients developed a new clinical phenotype thereafter. The associations between the different disease phenotypes are shown in Tables 3 and 4.
The associations between the cardiac, vascular and pulmonary manifestations in Behçet's disease patients.
| N=233N (%) | Cardiac5 (2.1%) | Pulmonary22 (9.4%) | Aortic disease6 (2.6%) | PAD12 (5.2%) | PVD62 (26.6%) | Vena caval disease17 (7.3%) | Superficial thrombophlebitis29 (12.4%) |
|---|---|---|---|---|---|---|---|
| Cardiac**5 (2.1%) | |||||||
| Yes | – | 4 | 1 | 0 | 1 | 1 | 1 |
| P | – | <0.001* | 0.12 | >0.999 | >0.999 | 0.32 | 0.49 |
| OR (95%CI) | – | 47 (5–440) | 11.2 (1–118.5) | – | 0.68 (0.08–6.2) | 3.3 (0.3–31.4) | 1.8 (0.2–16.6) |
| Pulmonary22 (9.4%) | |||||||
| Yes | 4 | – | 0 | 0 | 11 | 2 | 5 |
| P | <0.001* | – | >0.999 | 0.61 | 0.009* | 0.67 | 0.17 |
| OR (95%CI) | 47 (5–440) | – | – | – | 3.1 (1.3–7.7) | 1.3 (0.3–6.1) | 2.3 (0.8–6.8) |
| Aortic disease6 (2.6%) | |||||||
| Yes | 1 | 0 | – | 2 | 2 | 1 | 0 |
| P | 0.12 | >0.999 | – | 0.03* | 0.66 | 0.37 | >0.999 |
| OR (95%CI) | 11.2 (1–118.5) | – | – | 10.9 (1.8–66.4) | 1.4 (0.2–7.8) | 2.6 (0.3–24) | – |
| PAD12 (5.2%) | |||||||
| Yes | 0 | 0 | 2 | – | 9 | 1 | 1 |
| P | >0.999 | 0.61 | 0.03* | – | <0.001* | 0.61 | >0.999 |
| OR (95%CI) | – | – | 10.9 (1.8–66.4) | – | 9.5 (2.5–36.4) | 1.2 (0.1–9.6) | 0.6 (0.1–5) |
| PVD62 (26.6%) | |||||||
| Yes | 1 | 11 | 2 | 9 | – | 15 | 20 |
| P | >0.999 | 0.009* | 0.66 | <0.001* | – | <0.001* | <0.001* |
| OR (95%CI) | 0.68 (0.08–6.2) | 3.1 (1.3–7.7) | 1.4 (0.2–7.8) | 9.5 (2.5–36.4) | – | 27 (6–122.1) | 8.6 (3.6–20.2) |
| Vena caval disease17 (7.3%) | |||||||
| Yes | 1 | 2 | 1 | 1 | 15 | – | 8 |
| P | 0.32 | 0.67 | 0.37 | 0.61 | <0.001* | – | <0.001* |
| OR (95%CI) | 3.3 (0.3–31.4) | 1.3 (0.3–6.1) | 2.6 (0.3–24) | 1.2 (0.1–9.6) | 27 (6–122.1) | – | 8.3 (2.9–23.7) |
| Superficial thrombophlebitis29 (12.4%) | |||||||
| Yes | 1 | 5 | 0 | 1 | 20 | 8 | – |
| P | 0.49 | 0.17 | >0.999 | >0.999 | <0.001* | <0.001* | – |
| OR (95%CI) | 1.8 (0.2–16.6) | 2.3 (0.8–6.8) | – | 0.6 (0.1–5) | 8.6 (3.6–20.2) | 8.3 (2.9–23.7) | – |
PAD: peripheral arterial disease; PVD: peripheral venous disease,
The association between the constitutional manifestations and the musculoskeletal, neurological, ocular, cardiac, pulmonary and vascular systems involvement in Behçet's disease patients.
| N=233N (%) | Constitutional30 (12.9%) | MSK63 (27%) | Neurological59 (25.3%) | Ocular153 (65.7%) |
|---|---|---|---|---|
| Constitutional30 (12.9%) | ||||
| Yes | – | 7 | 12 | 16 |
| P | – | 0.62 | 0.048* | 0.13 |
| OR (95%CI) | – | 0.8 (0.3–2) | 2.2 (1–5) | 0.6 (0.3–1.2) |
| MSK63 (27%) | ||||
| Yes | 7 | – | 16 | 38 |
| P | 0.62 | – | 0.99 | 0.29 |
| OR (95%CI) | 0.8 (0.3–2) | – | 1 (0.5–2) | 0.7 (0.4–1.3) |
| Neurological59 (25.3%) | ||||
| Yes | 12 | 16 | – | 32 |
| P | 0.048* | 0.99 | – | 0.032* |
| OR (95%CI) | 2.2 (1–5) | 1 (0.5–2) | – | 0.5 (0.3–1) |
| Ocular153 (65.7%) | ||||
| Yes | 16 | 38 | 32 | – |
| P | 0.13 | 0.29 | 0.032* | – |
| OR (95%CI) | 0.6 (0.3–1.2) | 0.7 (0.4–1.3) | 0.5 (0.3–1) | – |
| Cardiac5 (2.1%) | ||||
| Yes | 4 | 0 | 2 | 1 |
| P | <0.001* | 0.33 | 0.6 | 0.05 |
| OR (95%CI) | 31.1 (3.3–288.7) | – | 2 (0.3–12.3) | 0.1 (0.01–1.1) |
| Pulmonary22 (9.4%) | ||||
| Yes | 8 | 2 | 2 | 10 |
| P | 0.003* | 0.05 | 0.07 | 0.036* |
| OR (95%CI) | 4.9 (1.9–13) | 0.2 (0.1–1.1) | 0.3 (0.06–1.2) | 0.4 (0.2–1) |
| Aortic disease6 (2.6%) | ||||
| Yes | 0 | 0 | 2 | 1 |
| P | >0.999 | 0.19 | 0.65 | 0.019* |
| OR (95%CI) | 1. | – | 1.5 (0.3–8.4) | 0.1 (0.01–0.9) |
| PAD12 (5.2%) | ||||
| Yes | 0 | 1 | 2 | 3 |
| P | 0.37 | 0.19 | 0.74 | 0.004* |
| OR (95%CI) | – | 0.2 (0.03–1.8) | 0.6 (0.1–2.7) | 0.2 (0.04–0.6) |
| PVD62 (26.6%) | ||||
| Yes | 7 | 11 | 12 | 31 |
| P | 0.83 | 0.05 | 0.21 | 0.002* |
| OR (95%CI) | 0.8 (0.3–2) | 0.5 (0.2–1) | 0.6 (0.3–1.3) | 0.4 (0.2–0.7) |
| Vena caval disease17 (7.3%) | ||||
| Yes | 2 | 4 | 5 | 8 |
| P | >0.999 | >0.999 | 0.77 | 0.09 |
| OR (95%CI) | 0.9 (0.2–4.1) | 0.8 (0.3–2.6) | 1.3 (0.4–3.7) | 0.4 (0.2–1.2) |
| Superficial thrombophlebitis29 (12.4%) | ||||
| Yes | 4 | 11 | 10 | 17 |
| P | >0.77 | 0.16 | 0.23 | 0.39 |
| OR (95%CI) | 1.1 (0.4–3.4) | 1.8 (0.8–4) | 1.7 (0.7–3.8) | 0.7 (0.3–1.6) |
MSK: musculoskeletal, PAD: peripheral arterial disease, PVD: peripheral venous disease.
In addition to the associations between the different disease phenotypes, further subgroup analysis was done giving rise to more associations. Regarding pulmonary involvement, pulmonary artery disease had an association with pulmonary hypertension (PH) (P<0.001) and pulmonary embolism (PE) (P<0.001); another association between PE and PH was detected (P=0.004). Concerning neurological system involvement, an association between dural sinus thrombosis and cerebrovascular disease (CVD) was detected (P=0.002). Furthermore, an association between dural sinus thrombosis and constitutional manifestations was detected (P=0.016). A number of associations were found concerning vascular involvement including an association between peripheral venous disease (PVD) and PE (P=0.009), and between superficial thrombophlebitis and PVD (P<0.001). Although the association between aortic involvement and CVD (carotid, vertebral and cerebral arteries involvement) was not statistically significant (P=0.09), it is considered clinically significant as two of six patients with aortic involvement had associated CVD [data available from the corresponding author on request].
Moreover, the different parts of a particular organ system tended to be concurrently affected; uveitis showed an association with retinitis (P<0.001) and retinal vasculitis (P<0.001). Furthermore, the coexistence of retinal vein thrombosis, and retinal vasculitis (P=0.007) and optic neuritis (P=0.017) was reported. Moreover, the different parts of the venous blood carrying system, the peripheral veins, pulmonary artery and right side of the heart, tend to be co-involved. Both the arterial and venous sides of the vasculature of an organ tended to be simultaneously affected; an association between dural sinus thrombosis and CVD was detected as mentioned earlier. Although no statistical significance could be detected, a clinical significance was evident as two of six patients with retinal artery thrombosis had concomitant retinal vein thrombosis [data available upon request].
DiscussionMucocutaneous manifestations were the most common onset features developing in 84.5% of the patients; they preceded the disease diagnosis by a mean duration of 8.4±27.8 months. Amongst them, oral ulcers were the most common (78.6%) followed by genital ulcers (64.3%). In agreement with our results, oral ulcers were reported to be the most common initial manifestation of BD in the Japanese,1 Turkish4 and Italian10 populations. The tendency of oral ulcers to precede the disease diagnosis has been reported,11 with a mean duration of −7.5±10.2 years.1 In contrast to these findings, Ideguchi and colleagues,2 and Alpsoy and coworkers4 reported that genital ulcers were the onset manifestation in only 16% and 14.2% of their Japanese and Turkish patients, respectively. In agreement with us, Ideguchi and coworkers reported that genital ulcers tend to predate the disease diagnosis with a mean of −1.5±5.4 years.1
Ocular manifestations were the onset manifestation in 14.6% of patients; they developed a mean of 18.2±42.9 months after diagnosis. Similar to our results, ocular manifestations were the initial manifestations in 14% of Japanese patients, with a mean duration of 1.1±4.6 years elapsing between diagnosis and ocular disease development1; a lower frequency was reported in Turkish patients (4.2%).4 Other Turkish3,6 and review12 studies reported the early development of the eye disease within the first four years following diagnosis.1
Neurological manifestations developed at the disease onset in 4.7% of the patients. A similar frequency was reported in a Japanese study (6%)13; while higher frequencies were reported in the Iraqi,14 Brazilian15 and Caucasian7 populations (10%, 16.7% and 23%, respectively). They had a tendency to develop a mean of 30.9±46 months after diagnosis. The late onset of neurological manifestations was also documented in several Japanese and Turkish studies; they tend to develop a range of four to ten years after diagnosis.1,3–5
Among the different patterns of vascular involvement, PVD was not only the most common pattern (26.6%) but also the earliest to occur; it developed as onset feature in 7.3% of patients and after a mean of 27.5±60.9 months following diagnosis. In accordance with our findings, Kural-Seyahi and colleagues reported that PVD was the presenting feature in 87% of patients with deep venous thrombosis; and it had a tendency to develop within the first two years after diagnosis.3
At onset, no patients presented with PAD or aortic disease; and only 0.4% and 1.3% of patients developed cardiac involvement and vena caval thrombosis at onset, respectively. They tended to develop a long period after diagnosis with mean periods of 47.3±63.9, 69.5±39.2 and 51.4±82.6 months for peripheral arterial, aortic involvement and vena caval disease, respectively. Cardiac involvement developed a mean of 11.8±25.9 months following diagnosis. The late development of PAD, aortic disease and vena caval thrombosis, a five- to ten-year after disease diagnosis, has been reported in studies from Japan,2 Turkey3,4 and the United States of America (USA).12 A late onset of cardiac involvement was reported in Israeli16 and Korean17 studies.
Pulmonary involvement was reported in 0.9% of the studied patients at disease onset. In a study performed by kural-Seyahi, one of ten patients with pulmonary arterial disease developed pulmonary aneurysms on presentation.3 On the other hand, BD and pulmonary aneurysms were diagnosed simultaneously in 2.4% of 210 patients presenting with pulmonary aneurysms supporting the rarity of pulmonary involvement at the disease onset.18 A mean duration of 44.5±54.4 months elapsed between criteria fulfilment and pulmonary disease development in this study that was nearly similar to other studies reporting median duration of 5 years and a mean period of 3.7±4.8 years after disease diagnosis in Turkish patients3 and another Egyptian cohort,19 respectively.
Only seven patients in this study had GIT disease that developed a mean of 101±87.8 months after diagnosis. In accordance with our results, GIT involvement was reported to be a late disease manifestation in studies from Japan and Turkey.1,3,4
Several associations between the different phenotypes were detected in this study. Ocular involvement had a negative association with the neurological disease, PVD, PAD, and aortic involvement. A negative association between the ocular disease and vascular manifestations was reported by Ideguchi and colleagues1 that is in agreement with the study results. In contradiction to our findings, an association between the neurological and ocular involvement was reported in studies from Japan,1 Brazil20 and USA.21
Associations between dural sinus thrombosis, and cerebrovascular disease (CVD) and constitutional features were observed in our cohort. In accordance with our findings, several authors from Turkey,3,5 United Kingdom7 and Iraq14 noticed that an active neurological disease is almost always associated with simultaneous constitutional manifestations. In agreement with our findings, no cases with concomitant dural sinus thrombosis and parenchymal neurological disease were found among a cohort of Turkish patients with neuro-Behçet's syndrome.5 Furthermore, another Turkish study reported that only 7% of patients with neuro-Behçet's syndrome had concomitant dural sinus thrombosis and parenchymal involvement.3
An association between PVD and pulmonary embolism (PE), superficial thrombophlebitis, vena caval thrombosis and PAD was observed in this study. Another association between vena caval thrombosis and superficial thrombophlebitis was also detected. Other studies from Turkey,3 Korea22 and India23 reported a link between PVD, and PAD and vena caval thrombosis which is consistent with our results. However, they also revealed other associations between PVD, and abdominal3,22,23 and pulmonary arterial18,24,25 diseases.
An association between cardiac involvement and constitutional features was detected. Another association between pulmonary involvement, and constitutional manifestations and cardiac involvement, particularly intracardiac thrombosis, was also detected. Similar to these findings, the association between intracardiac thrombosis and pulmonary aneurysms was reported in a Turkish study.26 Moreover, Edrees and colleagues reported that approximately one-third of their patients with pulmonary disease had concomitant fever.19 In addition, the coexistence of pulmonary aneurysms, vena caval involvement, intracardiac thrombosis and PAD was reported in Israeli18 and Turkish studies.24,25
It could take years for BD to evolve to the point of fulfilment of the classification criteria. In this study, a mean duration of 11.2±30,3 months elapsed between the disease onset and criteria fulfilment; however, this duration had a range extending up to 204 months. Similar to these findings, other studies1,4,21 reported durations of several years, with a mean duration of 4.3±5.7 years reported by Alpsoy and colleagues.4
Our data showed the tendency of some clinical phenotypes to evolve several years after diagnosis highlighting the restless nature of BD. Although some authors have reported that BD tends to abate over time,11,12 others from Iran,2 Turkey4,27 and Greece28 documented the unexpected course of BD. Moreover, the implementation of immunosuppressive therapy in young patients with a mild disease was suggested in studies from Turkey and Japan to modify the course of the disease.29,30
The limitations of the study include the small number of patients with the rare disease phenotypes e.g. aortic involvement, and its retrospective design.
In summary, the course of BD is unpredictable; it could take several years for the classification criteria to be fulfilled, thus, highlighting the importance of the close follow-up of patients with the serious disease characteristics but an incomplete presentation. The disease could evolve into another phenotype over several years calling into question the concept of a burnt-out disease. Moreover, BD tends to respect the anatomy of the involved organ system; it tends to involve the different parts of the affected organ system. Certain organ systems tend to be involved in association; this could help to predict organs that could be sub-clinically involved, and direct the physician towards the appropriate investigational plan.
FundingThis research did not receive any grant from any funding agencies.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsNone.