Calcium (Ca2+) is an important cation able to function as a second messenger in different cells of the immune system, particularly in B and T lymphocytes, macrophages, and mastocytes, among others. Recent discoveries related to the entry of Ca2+ through the store-operated calcium entry (SOCE) have opened a new investigation area about the cell destiny regulated by Ca2+ especially in B and T lymphocytes. SOCE acts through calcium-release-activated calcium (CRAC) channels. The function of CRAC depends upon two recently discovered regulators: the Ca2+ sensor in the endoplasmic reticulum or stromal interaction molecule (STIM-1) and one subunit of CRAC channels called Orai1.
This review focuses on the role of Ca2+ signals in B and T lymphocytes functions, the signaling pathways leading to Ca2+ influx, and the relationship between Ca2+ signals and autoimmune diseases.
El calcio (Ca2+) es un catión con capacidad multifuncional como segundo mensajero en diferentes grupos celulares del sistema inmunitario que incluyen los linfocitos T y B, los macrófagos, los mastocitos, entre otras. Los recientes descubrimientos en relación con la entrada de Ca2+ dependiente de depósito (SOCE por su sigla en inglés, store operated calcium entry) han abierto nuevos caminos en la investigación de cómo este catión dirige el destino celular, en especial en los linfocitos T y B. La SOCE actúa a través de canales CRAC (del inglés Ca2+ release-activated Ca2+ channels) y su mecanismo de activación depende de la interacción de dos moléculas reguladoras: un sensor del Ca2+ del retículo endoplásmico o molécula de interacción estromal (STIM-1, del inglés stromal interaction molecule) y una subunidad poro del canal CRAC (Orai1).
Esta revisión se centra principalmente en las funciones del Ca2+ en los linfocitos B y T, así como las alteraciones de estas vías implicadas en el desarrollo de enfermedades autoinmunes.
Calcium (Ca2+) is a multifunctional cation capable of acting as a second messenger in various immune cell groups including T and B lymphocytes, macrophages, mast cells, etc.1–3 Its distribution in intra-and extracellular spaces makes specialized pumps and channels necessary for its functioning and mobilization, as well as the influence of the cell depolarization or repolarization. Furthermore, the amount and duration of the flow of Ca2+ will determine the type and duration of its effect on intracellular signaling. Recent discoveries in connection with the reservoir dependent Ca2+ entry (SOCE by its acronym) have broken new ground in the research of how this cation directs cell fate, especially in T cells and B. This review focuses primarily on the roles of Ca2+in the last 2 cell groups and their involvement in autoimmunity.
Calcium as an Intracellular Signaling ElementFor normal cell function, especially regarding the immune system, the starting point is a basic principle consisting in the presence of the following elements:
- -
An external signal.
- -
A receptor.
- -
An internal signal, which depends on turn on the modifications undergone by the receptor in contact with the external signal and the cofactors that amplify the received signal.
- -
A transcription factor which moves to the nucleus.
- -
Transcription of ribonucleic acid (RNA) and translation to generate the protein(s) induced by the external signal.
Molecular biology studies and recent descriptions of4 new proteins have advanced the understanding of how these components of cell signaling work. External signals are formed by different proteins or peptide derivatives (cytokines, chemokines, pathogen associated peptides, etc.) which are picked up by a receptor located on the cell membrane. In the case of T and B lymphocytes, these receptors require the participation of other elements or co-receptors, which allow adequate extracellular signal synapses in order to activate the intracellular signaling cascade. The internal signal (or second messenger) is generated by multiple mechanisms (usually phosphorylation and dephosphorylation) through different routes, including JAK-STAT pathway MAP kinases, G-protein pathways, etc.5
Ca2+ acts as a second messenger and its importance is becoming more known thanks to recent findings regarding SOCE and its implications on the durability of the cellular responses to Ca2+ flux. Ca2+-induced signals were known for several decades, mainly due to their importance in RNA synthesis and cell division in leukocytes and thymocytes.6,7 Currently, there are various known functions dependent on the amount of Ca2+ present at the intracellular level. When Ca2+ levels increase during a short period they reduce lymphocyte mobility, thus enhancing the immunological synapse. In the case of other cell groups, high levels of short lived Ca2+ lead to other phenomena such as T cell-mediated cytotoxicity, release of lytic granules and/or cellular recognition processes as well as apoptosis. In contrast, when Ca2+ levels rise for a prolonged period, they can regulate transcriptional responses that will mark the cell fate of T and B lymphocytes (e.g. Cytokine production, cell differentiation, effector functions, non responsive states, etc.).8 These Ca2+ levels in lymphocytes are regulated dynamically through various channels, among which we find9:
- -
Intracellular channel receptors and the receptor for inositol-1,4,5-triphosphate (IP3) located in the sarcoplasmic reticulum.
- -
Plasma membrane channels, including Ca2+ release-activated Ca2+ chnnels (CRAC).
- -
Ca2+-dependent potassium channels activated by voltage.
- -
Energy dependent transporters channels.
- -
Nonselective cation channels.
- -
The sarco-endoplasmic reticulum adenosine triphosphatase pump (SERCA) and plasma membrane Ca2+ ATPase (PMCA).
In addition to these channels, the same concentration of intracellular Ca2+ entry helps regulate CRAC ion channels and the IP3 pathway, as well as the state of cell membrane repolarization. Once you increase the intracellular levels of Ca2+, this activates other signaling cascades that define the lymphocyte cell fate as reviewed below.
Calcium and Signaling in LymphocytesOnce the T or B lymphocyte is activated by its receptor and the process of costimulation occurs, recruitment, and activation of a group of protein tyrosine kinases, bound to other adapter proteins lead to the phosphorylation and activation of phospholipase C-γ (PLC γ 1 γ PLC in T and B lymphocytes 2). This enzyme, in turn, hydrolyzes phosphatidylinositol-3,4-bisphosphate (PIP2) from the cell membrane to 2 second messengers:
- -
IP3.
- -
Diacylglycerol (DAG).
IP3 then shifts and binds to its receptor on the membrane of the endoplasmic reticulum (ER) causing the release of Ca2+ stored inside and activation of other intracellular signals (Figs. 1 and 2). However, the rapid depletion of the ER Ca2+ leads to a short response. To facilitate the extension of cellular responses, another route of entry of Ca2+, called SOCE, is activated, which was recently discovered by large-scale searches of RNA interference. SOCE acts through CRAC channels and its mechanism of activation depends on the interaction of two regulatory molecules10:
- -
A Ca2+ sensor in the ER or stromal interaction molecule (STIM-1).
- -
A CRAC channel pore subunit (Orai1).
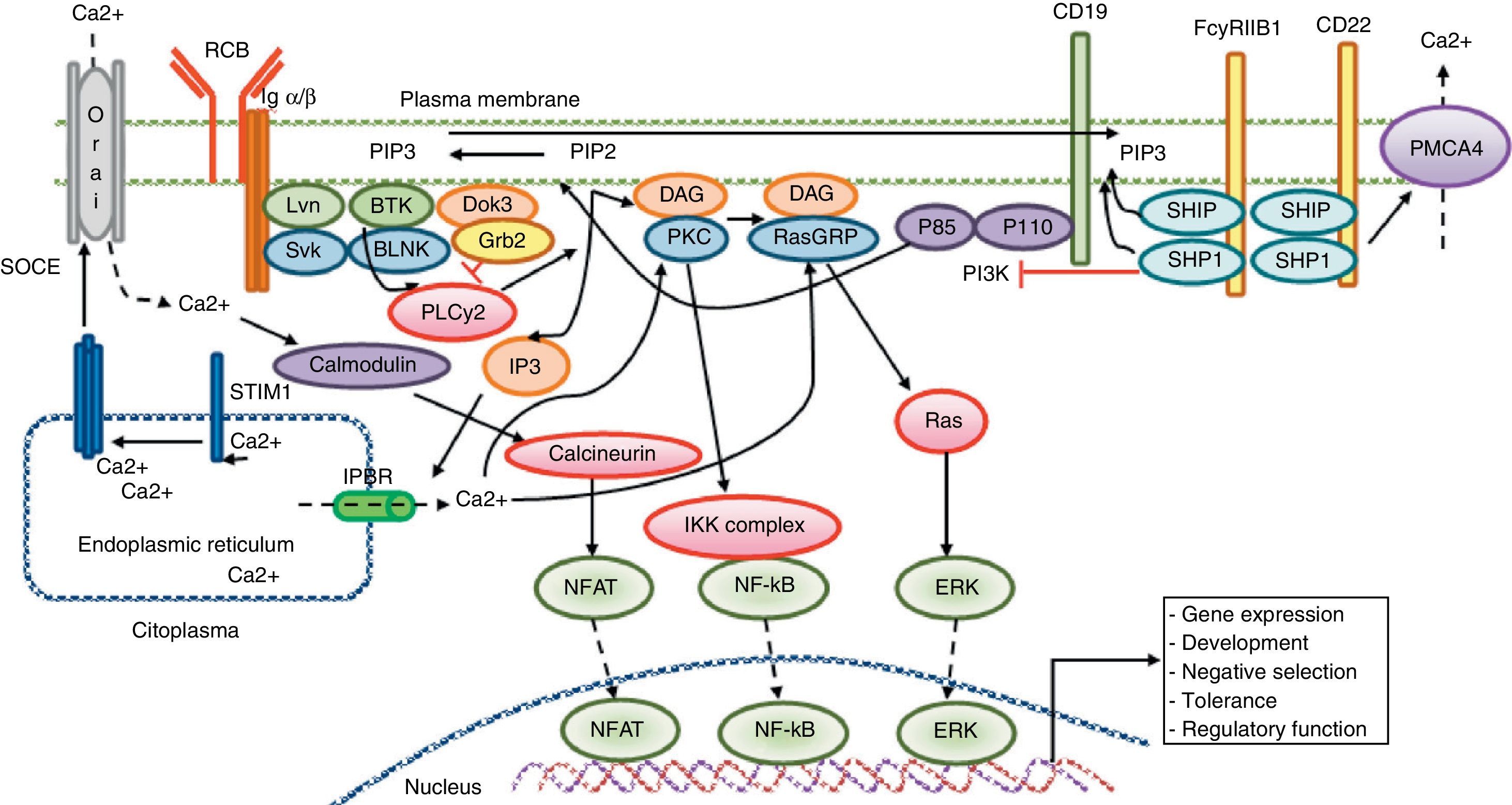
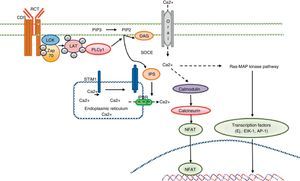
Calcium signaling in B lymphocyte. Antigen recognition by the B cell receptor activates different protein kinases, such as Lyn and Syk BLNK, leading to the activation of PLC γ 2, which hydrolyzes membrane bound PIP2 into small amounts of DAG and IP3. IP3 binds to its receptor IP3R located in the endoplasmic reticulum membrane and allows the release of stored calcium. Subsequently, the decrease in calcium level into the lumen of the endoplasmic reticulum generates the translocation of STIM1, which leads to the opening of the calcium channel to induce Orai1 dependent calcium entry from reservoir (SOCE). The CD19 receptor facilitates activation of the PI3K p110 subunit. This enzyme phosphorylates PIP2 to produce PIP3. The Fc receptor to low affinity IgG (FcgRIIB1) and CD22 are both negative regulators of calcium signaling during stimulation of B-cell receptor The negative function of these receptors is mediated by SHIP and SHP1. Additionally, CD22 inhibits signaling through calcium efflux mediated by PMCA4. Calcium entry generates activation of transcription factors responsible for the induction of new proteins responsible for the functions of proliferation, differentiation, and immune response.
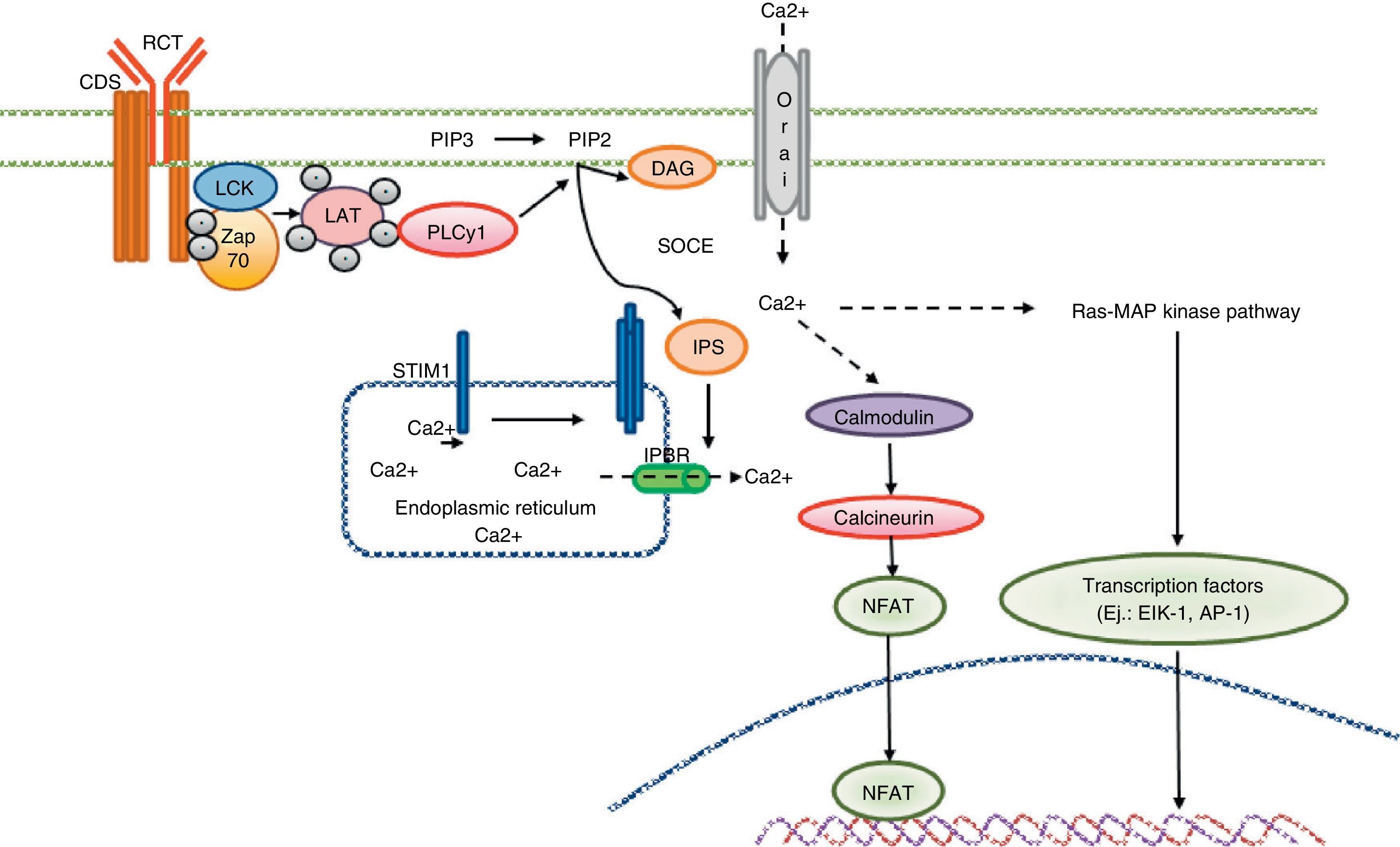
Calcium signaling in the T cell. Antigen recognition by the T cell receptor leads to tyrosine kinase activation inside the T cell, such as LCK and ZAP70, which results in phosphorylation and activation of γ PLC 1. PIP2 latter hydrolyzed in IP3 and DAG. The IP3 receptor opens the IP3R located in the endoplasmic reticulum and allows the output of calcium from the endoplasmic deposits. Calcium sensors detect STIM2 STIM1 and reducing calcium deposits by the N-terminal region of the lumen of the endoplasmic reticulum. STIM proteins are added in small groups in the endoplasmic reticulum membrane and produce extracellular calcium entry via the CRAC channel, Orai1. Intracellular calcium concentration activates the calcineurin pathway, NFAT, and the Ras-MAP kinase pathway.
The operation of this mechanism has been described thus: once the Ca2+ sensing STIM detects the decrease in the concentration of Ca2+ into the ER, it undergoes a conformational change (oligomerization and aggregation) forming a “point”, in juxtaposition to CRAC located as a transmembrane channel. This conformation makes contact with the Orai1 pore channel subunit, allowing the opening of the channel and the entrance of Ca2+. This increases the intracellular levels of the ion and perpetuates the responses generated by the activation of lymphocytes and their respective receptors. Other mechanisms have been described for regulating CRAC channel activity, including the activity of other Ca2+-permeable channels such as the TRPC family members, but this mechanism is not entirely clear. Also, other mechanisms have been postulated as regulators of STIM-Orai1 binding, where the cytoskeleton and the negatively charged membrane phospholipids can be an influence.11
Signaling Pathways Activated After Calcium InfluxOnce the increase in intracytoplasmic Ca2+ levels is achieved, they activate other signaling pathways and transcription factors that bind DNA and finally lead to the production of proteins, cytokines, etc. related to the inflammatory cascade. Within these pathways we find12–14:
- -
The calmodulin–calcineurin pathway, with the final activation of the nuclear factor of activated T cells (NFAT).
- -
The Ca2+-dependent kinase-calmodulin (CaMK) pathway, which has as transcription factors a protein bound to a response element ofcyclic adenosine monophosphate (CREB, cyclic-adesnosime monophosphate-responsive element binding protein) and myocyte enhancer factor 2.
- -
The nuclear factor κB (NF-κB).
Upon hydrolysis of PIP2, DAG is also obtained, which in turn can activate an additional two signaling pathways:
- -
The protein kinase C pathway (PKC), and the Ras-mitogen-activated protein kinase.
- -
The protein kinase C (PKC) pathway.
These pathways ultimately activate transcription factors such as AP-2 (a transcriptional complex formed by c-Fos and c-Jun) and NF-B. κ The various components and pathways related to the flow of Ca2+ are summarized in Table 1.
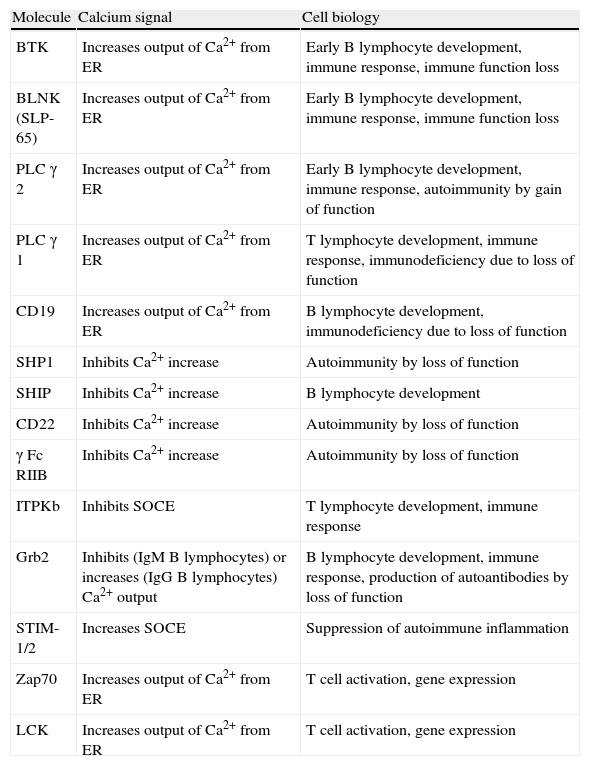
Calcium and Cell Function in B and T Lymphocytes.
| Molecule | Calcium signal | Cell biology |
| BTK | Increases output of Ca2+ from ER | Early B lymphocyte development, immune response, immune function loss |
| BLNK (SLP-65) | Increases output of Ca2+ from ER | Early B lymphocyte development, immune response, immune function loss |
| PLC γ 2 | Increases output of Ca2+ from ER | Early B lymphocyte development, immune response, autoimmunity by gain of function |
| PLC γ 1 | Increases output of Ca2+ from ER | T lymphocyte development, immune response, immunodeficiency due to loss of function |
| CD19 | Increases output of Ca2+ from ER | B lymphocyte development, immunodeficiency due to loss of function |
| SHP1 | Inhibits Ca2+ increase | Autoimmunity by loss of function |
| SHIP | Inhibits Ca2+ increase | B lymphocyte development |
| CD22 | Inhibits Ca2+ increase | Autoimmunity by loss of function |
| γ Fc RIIB | Inhibits Ca2+ increase | Autoimmunity by loss of function |
| ITPKb | Inhibits SOCE | T lymphocyte development, immune response |
| Grb2 | Inhibits (IgM B lymphocytes) or increases (IgG B lymphocytes) Ca2+ output | B lymphocyte development, immune response, production of autoantibodies by loss of function |
| STIM-1/2 | Increases SOCE | Suppression of autoimmune inflammation |
| Zap70 | Increases output of Ca2+ from ER | T cell activation, gene expression |
| LCK | Increases output of Ca2+ from ER | T cell activation, gene expression |
Signaling through intracellular Ca2+ has been implicated in the pathogenesis of autoimmune and congenital immunodeficiencies. Diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), multiple sclerosis (MS) and type 1 diabetes mellitus are related in their pathophysiology by the presence of autoreactive T and B cells that contribute in the generation of the inflammatory process. In SLE, autoreactive T cells replace the XΔ3-ζ chain with a ΦχP–γ common chain, leading to an intracellular signaling via the Syk (spleen tyrosine kinase) pathway and not ZAP-70, as is usual. This, associated with a group of lipid rafts in the plasma membrane of the T cell, increases intracellular Ca2+ in response to activation of the T cell receptor (TCR) by an autoantigen. The PKC pathway iv calmodulin-dependent (CAMK4) is activated, which binds to CREB and increases the production of interleukin 17 (IL-17) at the expense of the production of IL-2.15 This phenomenon has also been described in diseases such as RA and MS. However, in these conditions the proposed hypothesis is that the autoreactivity of the T cells due to mutations in the ZAP-70 kinase, markedly reduces TCR dependent signaling, including Ca2+ signals, allowing the autoreactive TCR to ‘escape’ negative selection in the thymus but become arthritogenic in peripheral tissues. This raises the issue of Ca2+ signaling participating in the selection process of thymic T cells.16 B cells, in the absence or lack of response to SHP1, CD22 or Fc γ RIIB1 generate increased responses to the influx of Ca2+ and, therefore, B lymphocyte hyperactivity and autoimmunity. In SLE, Fc γ RIIB1 defects are associated with increased response to Ca2+ in B lymphocytes.17 Although diseases such as SLE are characterized by a systemic component, alterations in calcium signaling have been described in T and B lymphocytes, and have not been associated with susceptibility to specific manifestations of the disease (e.g. increased risk of nephritis). However, derived from the murine OraiK1/K1 model (discussed below), the lack of calcium signaling in immune cells protects against the development of colitis in the transfer model of inflammatory bowel disease.
As mentioned previously, in T cells, the Ca2+ entry following antigenic stimulation is essential for the activation of NFAT. It has been demonstrated, in a group of patients with mutations in either STIM 1 u Orai1, that the affected protein function or expression results in a defect of stored Ca2+ influx and CRAC channel function, resulting in decreased lymphocyte activation These mechanisms lead to the presentation of an inherited form of severe combined immunodeficiency, demonstrating the importance of these channels in the normal function of T-lymphocytes in humans. Similarly, calcium signaling defect can lead to autoreactive T cells to a state of activation which can result in the development of autoimmune diseases. Given the role of calcium in the activation of autoimmune phenomena, Lin et al. have recently published the generation of humanized monoclonal antibodies directed against high affinity human Ora1. These antibodies showed decreased Ca2+ entry, NFAT transcription and release of cytokines and may represent a new therapeutic target in the treatment of autoimmune diseases.18 This approach affects different tissues involving calcium signaling, and different immune system effects should be better understood.
In an animal model developed by McCarl et al. the role of Orai1 was described in autoimmunity. In this study, T and B lymphocytes derived from a mouse model of OraiK1/K1 expressed a mutated nonfunctional Orai1 protein. These cells showed a severe decrease in the influx of Ca2+ and CRAC channel function, resulting in decreased expression of several cytokines, including IL-1, IL-4, IL-17, interferon gamma and tumor necrosis factor (TNF) alpha in CD4 and CD8 T lymphocytes. This model showed greater tolerance to skin grafts compared with animals without the mutation and failed to develop colitis type autoimmune phenomena. These findings confirm the importance of these channels and the influx of Ca2+ on the activation of the adaptive immune system cells.19
Ca2+ is also involved in the development of congenital immunodeficiencies. In common severe immunodeficiency, for example, there is a mutation in the T cell, more specifically in the transmembrane domain of Orai1 in CRAC, which allows the entry of Ca2+ via SOCE, giving rise to T lymphocytes with low proliferative capacity and cytokine production. This defect may also affect SOCE B cells and fibroblasts in this disease.20 In X-linked a gammaglobulinemia presents an inherited deficit in B cells, which is characterized by mutations in Bruton tyrosine kinase, the enzyme responsible for the activation of PLC γ 2 with the effect of reducing the Ca2+ entry pathway mediated by SOCE due to21 low IP3 generation. Finally, common variable immunodeficiency also presented alterations in the functioning of B lymphocytes, especially mutations in CD19, decreasing Ca2+ influx into the cell, compromising the response to antigens and reducing CD27 positive B cell generation.22
ConclusionsIntracellular Ca2+ signaling is a component of signaling cascades that are generated in different cells after exposure of its receptors to a specific stimulus. In the case of T and B lymphocytes, these signals give rise to multiple changes in intracellular DNA expression, with the consequent production of various inflammatory markers. Research in this area also has established the importance of Ca2+ pathways in the development of autoimmune diseases, both due to overproduction as well as inactivity. As progress is made in the study of all these concepts, it becomes important to consider intervention on these pathways and receptors for therapeutic purposes.
Ethical ResponsibilitiesProtection of people and animalsThe authors declare that experiments have not been performed on humans or animals.
Data confidentialityThe authors state that no patient data appear in this article.
Right to privacy and informed consentThe authors state that no patient data appear in this article.
Conflict of InterestThe authors declare no conflict of interest.
Please cite this article as: Izquierdo JH, Bonilla-Abadía F, Cañas CA, Tobón GJ. Calcio, canales, señalización intracelular y autoinmunidad. Reumatol Clin. 2014;10:43–47.