Ultrasound (US) remission in rheumatoid arthritis (RA) targets synovitis absence. Tenosynovitis triggers flares. Despite increased ultrasound use, flare patterns among patients with low disease activity (LDA) and ultrasound remission, especially in real-world settings, are poorly understood. This study examined flare rates and predictors of US remission in patients without synovitis or tenosynovitis.
Materials and methodsIn a study of 88 patients achieving US remission and LDA, the focus was on the time to the first flare over a 2-year follow-up. US remission, indicated by the absence of active synovitis and tenosynovitis based on a power Doppler (US-PD) score of 0, was assessed on various joints. Flares are defined by the need for additional medication or encountering a US-PD flare. They were monitored at the baseline, 1-year, and 2-year visits with further US evaluation at clinical flare-ups. Baseline factors linked to a shorter time to flare were analyzed.
ResultsAt 1 year, LDA and US remission rates were 75% and 92%, respectively, and at 2 years, 73% and 87% respectively. Over the 2 years, 40% experienced flare, occurring on average at 11.7±7.0 months. Notably, 5.7% have US-PD flares without clinical signs. Analysis revealed Stage III disease and CRP as factors linked to a shorter time to flare.
Discussion and conclusionsIn patients with RA achieving LDA and US remission, frequent flares were observed with US remission over 2 years, but most maintained sustained remission. Baseline factors are essential for predicting flares, emphasizing continuous monitoring and personalized treatment to sustain remission and minimize flare risks in RA management.
La remisión por ultrasonido (US) en la artritis reumatoide (AR) busca la ausencia de sinovitis y tenosinovitis, cuya presencia puede desencadenar brotes. Este estudio analiza las tasas de brotes y sus predictores en pacientes con baja actividad de la enfermedad (ABE) y remisión por US.
Materiales y métodosSe siguió a 88 pacientes con remisión por US y ABE durante dos años, evaluando el tiempo hasta el primer brote. Los brotes se definieron por la necesidad de medicación adicional o por la detección de brotes de US-PD (ultrasonido-Power Doppler). Se realizaron evaluaciones de US al inicio, y a los uno y dos años, además de durante los brotes clínicos.
ResultadosAl año, 75% de los pacientes mantuvieron ABE y 92% remisión por US; a los dos años, estos porcentajes fueron 73% y 87%, respectivamente. El 40% experimentó brotes, generalmente a los 11,7±7,0 meses. Un 5,7% presentó brotes de US-PD sin síntomas clínicos. La etapa III y la proteína C-reactiva (CRP) fueron identificadas como factores de riesgo para un brote más temprano.
Discusión y conclusionesA pesar de los frecuentes brotes, la mayoría de los pacientes con AR mantuvieron remisión sostenida durante los dos años de estudio. Los factores basales son cruciales para predecir brotes, lo que subraya la importancia del monitoreo continuo y un tratamiento personalizado para mantener la remisión y reducir el riesgo de brotes en el manejo de la AR.
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by persistent synovitis, which can lead to joint destruction.1 As emphasized by the treat-to-target (T2T) recommendations,2 the treatment goal for patients with RA is to suppress inflammation and achieve and sustain low disease activity (LDA) or, ideally, clinical remission (CR). This is assessed using composite disease activity measures such as the disease activity score based on 28-joint counts (DAS28), the Clinical Disease Activity Index (CDAI), and the Simplified Disease Activity Index (SDAI). Composite disease activity measures are widely accepted as optimal treatment targets in daily clinical care. However, they do not consider subclinical inflammation. Flares are frequent in patients with RA in CR, with 30–50% of patients experiencing a disease flare within the first 2 years.3
Recent studies have highlighted that ultrasound (US)-detected residual synovitis is prevalent in patients with RA in CR and has been shown to predict flares and structural progression.4 Therefore, imaging has gained significant importance in the assessment of RA disease activity. Particularly, US has several advantages over magnetic resonance imaging (MRI), such as lower cost and better accessibility in clinical practice. Ultrasound-power Doppler (US-PD) measures the amplitude of flow signals in blood vessels with high sensitivity and has been shown to reflect disease activity.5 Several studies have suggested that PD-positive findings in at least one joint are the main predictors of flares and that PD-negative joints are less likely to flare for a certain period, regardless of whether treatment was initiated or not.6,7
Thus, US remission is generally perceived as a disease state in which no synovial inflammation is visualized based on PD activity, and it is thought to more accurately define the remission state than clinical assessment alone.8,9 Recently, US assessment has gained popularity among rheumatologists worldwide for the diagnosis of imaging remission and monitoring disease activity in daily clinical care.10 Additionally, the on-demand use of US assessment in the most symptomatic joint alongside routine examination is useful in the management of RA.11
Most previous studies on RA flares have focused on cohorts with LDA or CR assessed based on composite disease activity measures. However, few studies have examined patients with US readmission using US as a daily rheumatologic practice throughout the follow-up period, especially prospective studies in real-world settings. Questions remain regarding the frequency of flares, the timing of flare occurrence, and flare predictors in patients with RA who have achieved US remission. To date, there is no consensus regarding the definition of US remission. Previous studies primarily excluded intra-articular synovitis in defining US remission, but PD-positive tenosynovitis is a risk factor for flares.12–14 Therefore, a study is needed to define US remission as the complete absence of both active joint synovitis and tenosynovitis.
The overall aim of this study in patients with RA who achieved both CR/LDA and US remission with routine US monitoring was twofold: (1) to investigate the flare rate and clinical course within a 2-year follow-up period and (2) to identify baseline predictive factors for flares.
Materials and methodsStudy designThis prospective observational study investigated patients with RA who achieved both US remission and LDA during a 2-year follow-up period under daily US monitoring. Throughout the 2-year follow-up period, we evaluated (1) the clinical course, including flare rate and time to flare; (2) sustained US remission and/or low disease activity at the 1- and 2-year follow-up visits; and (3) baseline predictors for the flares. This study was conducted at two hospitals, the first hospital between April 2016 and November 2020 and the second hospital between April 2020 and April 2023, as part of the patient's routine care.
PatientsConsecutive patients with RA who were followed up at both institutions were recruited through the clinical practice of rheumatologists. All patients met the following criteria: (1) confirmed RA classified according to the American Rheumatism Association 1987 revised criteria15 and/or the American College of Rheumatology/European League against Rheumatism classification 2010 criteria for RA16; (2) age >18 years; (3) at least 12 months disease duration; (4) maintaining the same RA treatment at least 6 months; (5) being in sustained LDA or CR according to either DAS28 CRP or SDAI/CDAI for at least one month apart; (6) being in US remission. Patients with missing data at the 1-year or 2-year follow-up and those who dropped out of follow-up within 2 years were excluded.
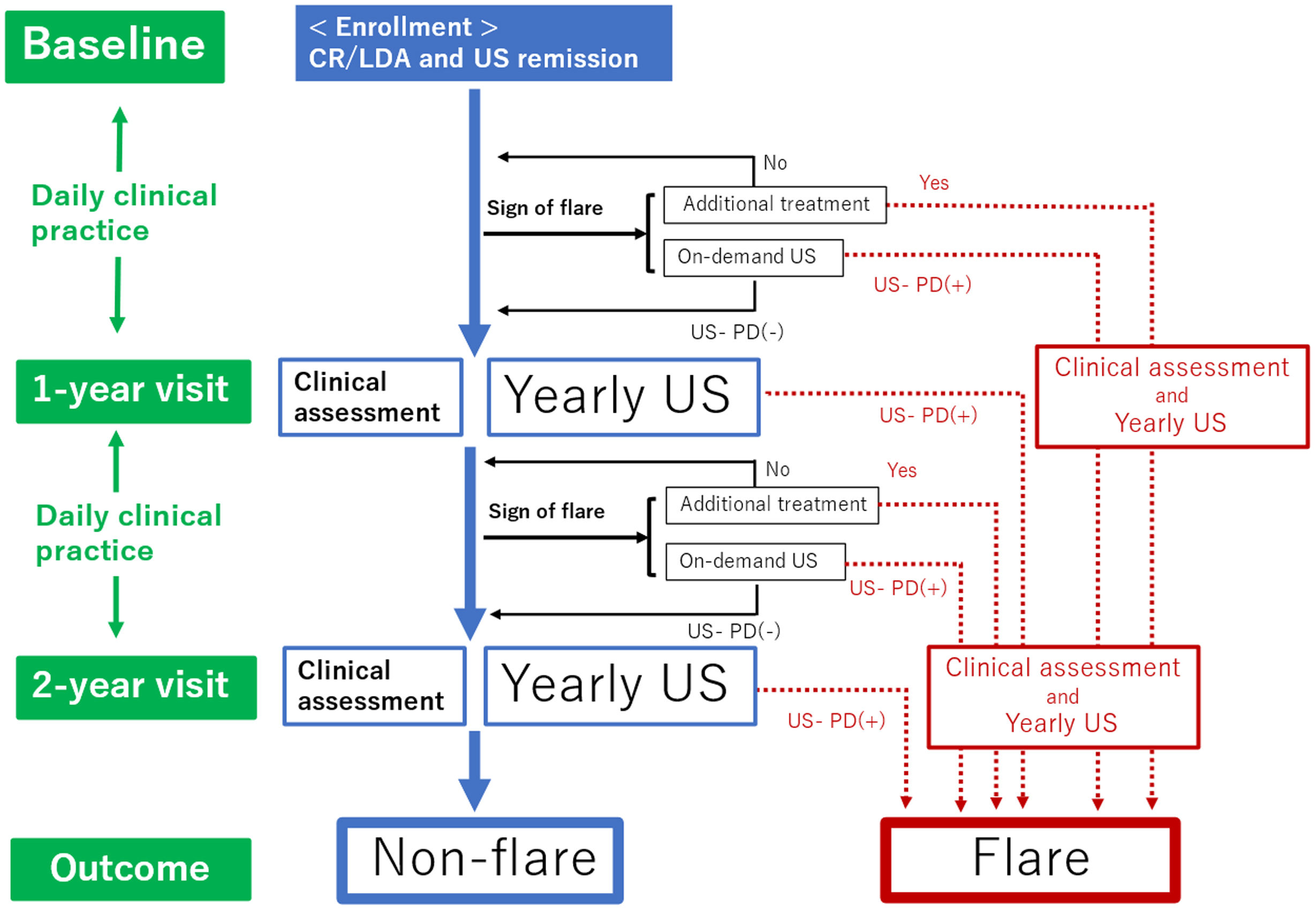
Follow-upAs shown in Fig. 1, all patients were followed up for two years from baseline. The clinical follow-up visit schedule and therapy were determined by the treating rheumatologist based on the disease evolution of each patient in real-world scenarios. The study examined two follow-up visits: one at 1-year and another at 2 years visit (with a ±1 month allowance). These follow-up visits were conducted regardless of flare occurrence, with data from both clinical assessments and US examinations being recorded. Follow-ups regarding the patient's electronic medical history were performed through consultations. During the study period, patients were routinely assessed by their rheumatologists and, in cases of worsening RA, at any time between clinical visits. The decision to change the disease-modifying anti-rheumatic drug (DMARD) therapy and order on-demand US was made by the patients’ consultant rheumatologist, according to their clinical practice, based on a benefit analysis as well as informed and shared decision-making.
Flow chart of this study. Time periods are shown on the left side. Clinical practice continues daily throughout the 2-year study period. Clinical assessments and yearly US assessments are conducted at the 1-year and 2-year follow-up visits. When the patients encounter additional treatment or US-PD flares according to the definition of flares in the case patient shows the sign of a flare, they will be divided into flare groups. Patients who do not meet the definition of flare through the observation period will be divided into the non-flare group. CR: clinical remission; LDA: low disease activity; US: ultrasound; US-PD: ultrasound-power Doppler.
During the 2-year follow-up, clinical assessment data and US assessments were collected at baseline and at the 1-year and 2-year visits as part of routine care. Any modifications in therapy and US assessment were also recorded during the follow-up period. Treatment tapering was defined as reducing the dose or discontinuing the DMARD and prednisolone (PSL) for therapeutic relief within the first 1 year from baseline. Cases in which a patient experienced a flare before treatment tapering were excluded from this definition.
Clinical and laboratory assessmentThe following data were collected at baseline: age, sex, body mass index (BMI), musculoskeletal comorbidities, disease duration, Steinbrocker stage within the past 3 months, and various laboratory markers such as rheumatoid factor (RF), anti-citrullinated protein antibody (ACPA) at diagnosis, albumin, hemoglobin, erythrocyte sedimentation rate (ESR), C reactive protein (CRP), and matrix metalloproteinase-3 (MMP-3). Physical function was evaluated at baseline using the Health Assessment Questionnaire Disability Index (HAQ-DI).17
Psychiatric factors are of interest because of their association with RA flares.18 Questionnaires were collected at baseline to assess psychiatric disorders and pain catastrophizing. The presence of psychiatric disorders was evaluated using the Brief Scale for Psychiatric Problems in Orthopedic Patients (BS-POP).19 This is an established scale that can detect psychiatric problems, such as depression and sleep disorders, as well as the overall psychiatric condition and linked disorders (i.e., musculoskeletal disorders). Pain catastrophizing was evaluated using the Pain Catastrophizing Scale (PCS),20 with scores ranging from 0 to 52. Higher scores corresponded to higher levels of pain catastrophizing. In this study, we used the Japanese version of the PCS,21 which was supported by a confirmatory factor analysis. At baseline, as well as at the 1-year and 2-year visits, the following data were collected: treatment information, patient visual analog scale (VAS) scores, patient global assessment (PGA), evaluator global assessment (EGA), 28 tender joint counts (TJC), swollen joint count (SJC), DAS28, CDAI, and SDAI.
Additionally, we evaluated the tender-swollen joint count difference (TSJD) and the difference between the PGA and EGA as potential predictors of flares because of their significance.22,23
US assessmentUS assessment was performed at baseline, at the 1-year visit, and at the 2-year visit (yearly US assessment), and the presence of findings recorded. All assessments were performed on the same visit day as the clinical assessment. Additionally, the US data was assessed based on clinical decisions by consulting a rheumatologist whenever patients showed clinical manifestations of a flare at any time (on-demand US assessment).
US examination was performed using a high-sensitivity ultrasound equipment Noblus device (Hitachi Medical Corporation, Tokyo, Japan), LOGIQ e Premium, and Venue Go (GE Healthcare, IL, USA). These devices were equipped with high-frequency (12–20MHz) linear transducers and utilized a high-definition dynamic tissue harmonic imaging-penetration setting. The pulse repetition frequency was set between 500 and 1000Hz, and the Doppler frequency ranged from 6.1 to 10.0MHz, with adjustments made according to the device used. Six rheumatologists with professional training and experience in musculoskeletal ultrasonography performed the examinations in accordance with the guidelines recommended by the Japan College of Rheumatology (JCR).24 Six rheumatologists with professional training and experience in musculoskeletal US performed the examinations in accordance with the guidelines recommended by the Japan College of Rheumatology (JCR). Of these six rheumatologists, three were registered sonographers under the JCR, while the remaining three performed the US evaluations under their supervision. The sonographers were not blinded to the clinical data.
The presence of joint synovitis was evaluated using bilateral metacarpophalangeal (MCP) 1–5, proximal interphalangeal (PIP/IP) 1–5, and wrist joints using longitudinal and transverse scans from the dorsal side, following the recommendations provided by the Outcome Measures in Rheumatology in Clinical Trials.25 The PD signals for synovial vascularization were scored semi-quantitatively based on the atlas set forth by the JCR.24 Active joint synovitis was defined as the presence of a positive PD signal within any joint. Tenosynovitis was evaluated in the 1st through 6th components of the carpal extensor tendon bilaterally. Active tenosynovitis was defined as the presence of a positive PD signal within any tendon.25
In this study, we routinely screened the hand joints based on previous reports indicating the efficacy of hand screening, which greatly reflects other joints, including the ankle, elbow, and knee joints.26–28 Additionally, we examined the tender or swollen joints to screen for active synovitis and tenosynovitis. US remission was defined as the absence of active joint synovitis or tenosynovitis in any evaluated joint or tendon. In cases where a patient showed active joint synovitis or tenosynovitis in any joint or tendon during yearly US assessment or on-demand US evaluations, we defined it as a US-PD flare.
Definition of flareFlare was defined in the following situations: (1) when patients received additional DMARDs or glucocorticoids due to any increase in disease activity or flare necessitating increased medication or (2) when patients experienced a US-PD flare. Flares have been defined in previous studies based on patient-reported outcomes, questionnaires, and increased disease activity scores assessed using composite measures. When an increase in disease activity is observed, flares are typically managed with the intensification of treatment. In this study, a flare was defined as an increase in medication prescribed by the physician, with an emphasis on real-life settings. In addition to obvious flares that require intensified medication, patients that experience subjective symptoms are often encountered in daily clinical practice, making it difficult to decide if a flare has occurred. However, when the US is performed, active synovitis is identified. Therefore, US flare was also included in the definition of flares, with an emphasis on monitoring the maintenance of US remission.
Outcome measuresThe primary outcome was the time from baseline to the occurrence of the first flare. In cases where patients experienced multiple flares, only the first flare was considered. The secondary outcomes included the remission rate, assessed through DAS28 CRP, SDAI/CDAI, and US remission at the 1-year and 2-year follow-up visits. Additionally, the rate of US-PD flares at the yearly US assessment (yearly US-PD flare) and changes in ongoing medications were also recorded.
Statistical analysisDemographic and descriptive continuous variables are expressed as the mean and standard deviation (SD) when normally distributed. For non-normally distributed data, variables are expressed as the median and interquartile range (IQR), while categorical variables are expressed as counts and percentages. The statistical difference in the change of methotrexate (MTX) and PSL dose from baseline to the 1-year and 2-year follow-up visits was determined using a two-sided paired t-test.
When comparing variables between the non-flare and flare groups, the Mann–Whitney U test was utilized for non-normally distributed data or the independent sample t-test was employed for normally distributed data to compare continuous variables. For categorical variables, Fisher's exact test was used for non-normally distributed data, while the chi-squared test was used for normally distributed data. The baseline factors associated with a shorter flare time were analyzed using univariate COX regression analysis. Treatment tapering within 1 year, which increased the flare rate,29 was included in the analysis. Among the factors identified in the univariate analysis, multivariate COX regression analysis was performed to identify factors that were independently associated with flares. The results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs).
The cutoff and area under the curve (AUC) values were calculated using receiver operating characteristic curves for continuous variables that showed significant differences in the multivariate analysis. A significance level of p<0.05 was used for all analyses. All statistical analyses were performed using EZR software.30
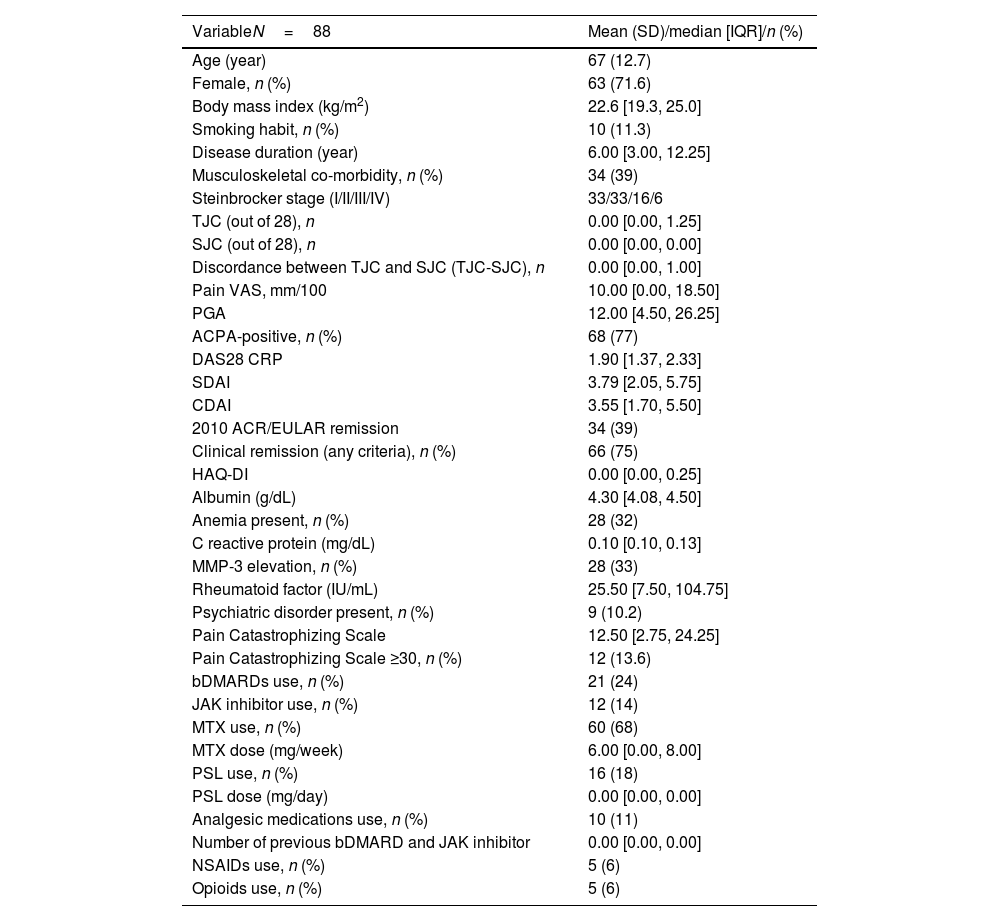
ResultsPatient characteristicsA total of 107 patients were enrolled at baseline. Among them, 10 were lost to follow-up, and nine were excluded because of missing data at the two follow-up visits. Therefore, 88 patients were included in the study. The baseline demographic and clinical characteristics are depicted in Table 1. The median disease duration was 6 years, indicating a relatively long-standing RA in this sample. Sixty-six patients (75%) were classified as Stage I/II according to the Steinbrocker staging, suggesting early to moderate joint destruction. No cases of orthopedic surgery were included in this study. The median TJC was 0, and the median pain VAS score was 10, indicating a relatively low pain intensity. Sixty-six patients (75%) fulfilled at least one of the clinical remission criteria according to the DAS28 CRP, SDAI, or CDAI, while 34 patients (38.6%) fulfilled the ACR/EULAR 2010 remission criteria.31 The median HAQ-DI was 0.0, indicating a relatively low level of physical disability. Psychiatric disorders, as assessed by the BS-POP, were observed in nine patients (10.2%). A PCS score ≥30 was found in 12 patients (13.6%).
Baseline demographic and clinical characteristics of the patients.
| VariableN=88 | Mean (SD)/median [IQR]/n (%) |
|---|---|
| Age (year) | 67 (12.7) |
| Female, n (%) | 63 (71.6) |
| Body mass index (kg/m2) | 22.6 [19.3, 25.0] |
| Smoking habit, n (%) | 10 (11.3) |
| Disease duration (year) | 6.00 [3.00, 12.25] |
| Musculoskeletal co-morbidity, n (%) | 34 (39) |
| Steinbrocker stage (I/II/III/IV) | 33/33/16/6 |
| TJC (out of 28), n | 0.00 [0.00, 1.25] |
| SJC (out of 28), n | 0.00 [0.00, 0.00] |
| Discordance between TJC and SJC (TJC-SJC), n | 0.00 [0.00, 1.00] |
| Pain VAS, mm/100 | 10.00 [0.00, 18.50] |
| PGA | 12.00 [4.50, 26.25] |
| ACPA-positive, n (%) | 68 (77) |
| DAS28 CRP | 1.90 [1.37, 2.33] |
| SDAI | 3.79 [2.05, 5.75] |
| CDAI | 3.55 [1.70, 5.50] |
| 2010 ACR/EULAR remission | 34 (39) |
| Clinical remission (any criteria), n (%) | 66 (75) |
| HAQ-DI | 0.00 [0.00, 0.25] |
| Albumin (g/dL) | 4.30 [4.08, 4.50] |
| Anemia present, n (%) | 28 (32) |
| C reactive protein (mg/dL) | 0.10 [0.10, 0.13] |
| MMP-3 elevation, n (%) | 28 (33) |
| Rheumatoid factor (IU/mL) | 25.50 [7.50, 104.75] |
| Psychiatric disorder present, n (%) | 9 (10.2) |
| Pain Catastrophizing Scale | 12.50 [2.75, 24.25] |
| Pain Catastrophizing Scale ≥30, n (%) | 12 (13.6) |
| bDMARDs use, n (%) | 21 (24) |
| JAK inhibitor use, n (%) | 12 (14) |
| MTX use, n (%) | 60 (68) |
| MTX dose (mg/week) | 6.00 [0.00, 8.00] |
| PSL use, n (%) | 16 (18) |
| PSL dose (mg/day) | 0.00 [0.00, 0.00] |
| Analgesic medications use, n (%) | 10 (11) |
| Number of previous bDMARD and JAK inhibitor | 0.00 [0.00, 0.00] |
| NSAIDs use, n (%) | 5 (6) |
| Opioids use, n (%) | 5 (6) |
SD: standard deviation; IQR: interquartile range; TJC: tender joints count; SJC: swollen joints count; VAS: Visual Analogue Scale; PGA: patient global assessment; ACPA: anti-citrullinated protein/peptide antibody; DAS28: 28-joint count disease activity score; SDAI: simplified disease activity; CDAI: clinical disease activity; ACR: American College of Rheumatology; EULAR: European League Against Rheumatism; HAQ-DI: Health Assessment Questionnaire Disability Index; MMP-3: matrix metalloproteinase-3; bDMARD: biological disease-modifying anti-rheumatic drug; JAK: Janus kinase; MTX: methotrexate; PSL: prednisolone.
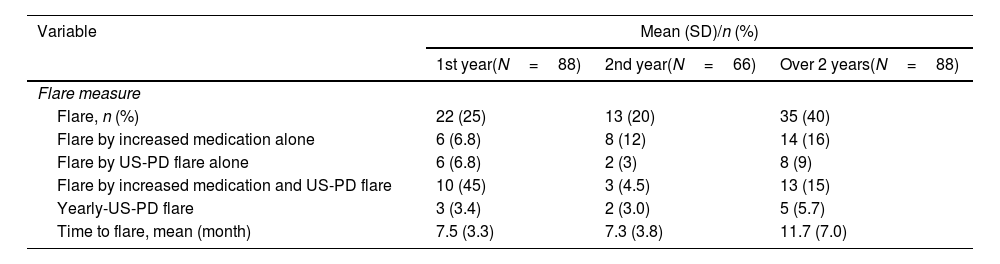
Table 2 shows the clinical courses of the 88 patients included in this study. Over the 2 years, 35 patients (40%) experienced a flare, and the mean time to flare was 11.7±7.0 months. Flares due to increased medication were reported in six patients (6.8%), while US-PD flares were observed in 19 patients (22%) from baseline to the 1-year follow-up visit. Among the 66 patients who had not experienced flares from baseline to the 1-year follow-up visit, eight (12%) had flares with increased medication, and 11 (17%) had US-PD flares during the 1-year visit to the 2-year visit. During the yearly US assessment, three patients (3.4%) at the 1-year follow-up visit and two patients (3.0%) at the 2-year follow-up visit had US-PD flares, despite having no clinical signs of flares. Among the three patients who exhibited US flares at the 1-year visit, one patient received additional treatment just after the US assessment, while the remaining two patients did not receive additional treatment at that time. However, one patient did receive additional treatment during the follow-up period of the study.
Clinical course of the 88 patients included in the study.
| Variable | Mean (SD)/n (%) | ||
|---|---|---|---|
| 1st year(N=88) | 2nd year(N=66) | Over 2 years(N=88) | |
| Flare measure | |||
| Flare, n (%) | 22 (25) | 13 (20) | 35 (40) |
| Flare by increased medication alone | 6 (6.8) | 8 (12) | 14 (16) |
| Flare by US-PD flare alone | 6 (6.8) | 2 (3) | 8 (9) |
| Flare by increased medication and US-PD flare | 10 (45) | 3 (4.5) | 13 (15) |
| Yearly-US-PD flare | 3 (3.4) | 2 (3.0) | 5 (5.7) |
| Time to flare, mean (month) | 7.5 (3.3) | 7.3 (3.8) | 11.7 (7.0) |
| Variable | 1-Year visit(N=88) | 2-Year visit(N=88) |
|---|---|---|
| Clinical measure | ||
| Low disease activity, n (%) | 81 (92) | 81 (92) |
| Clinical remission, n (%) | 66 (75) | 64 (73) |
| US remission, n (%) | 81 (92) | 76 (87) |
| bDMARDs use, n (%) | 19 (22) | 21 (23) |
| JAK inhibitor use, n (%) | 12 (14) | 12 (14) |
| MTX use, n (%) | 54 (61) | 48 (55) |
| MTX dose, mg/w | 7.0 (2.6) | 6.8 (2.8) |
| ΔMTX dose from BL, mg/w | −0.9 (2.1) | −1.5 (2.7) |
| PSL use, n (%) | 17 (19) | 10 (11) |
| PSL dose, mg/d | 4.0 (1.5) | 4.3 (1.8) |
| ΔPSL dose from BL, mg/day | 0.1 (1.6) | −1.7 (1.7) |
SD: standard deviation; BL: baseline; US-PD: ultrasound-power Doppler; bDMARD: biological disease-modifying anti-rheumatic drug; JAK: Janus kinase; MTX: methotrexate; PSL: prednisolone.
At the 1-year visit, LDA, CR, and US remissions were observed in 81 (92%), 66 (75%), and 81 (92%) patients, respectively. At the 2-year visit, these remission rates were observed in 81 (92%), 64 (73%), and 77 (87%) patients, respectively. The use of biologic DMARDs and Janus kinase (JAK) inhibitors remained unchanged between baseline, the 1-year visit, and the 2-year visit, whereas the use of MTX and PSL decreased. The ΔMTX and ΔPSL doses compared to baseline were −0.9 (2.1) mg/week (p<0.001) and 0.1 (1.6) mg/day (p=0.48) at the 1-year visit and −1.5 (2.7) mg/week (p<0.001) and −1.7 (1.7) mg/day (p=0.35) at the 2-year visit. Among the 22 patients who experienced flares within the first year, 13 (59%) achieved LDA and US remission at the 1-year visit, and 16 (73%) achieved LDA and US remission at the 2-year visit.
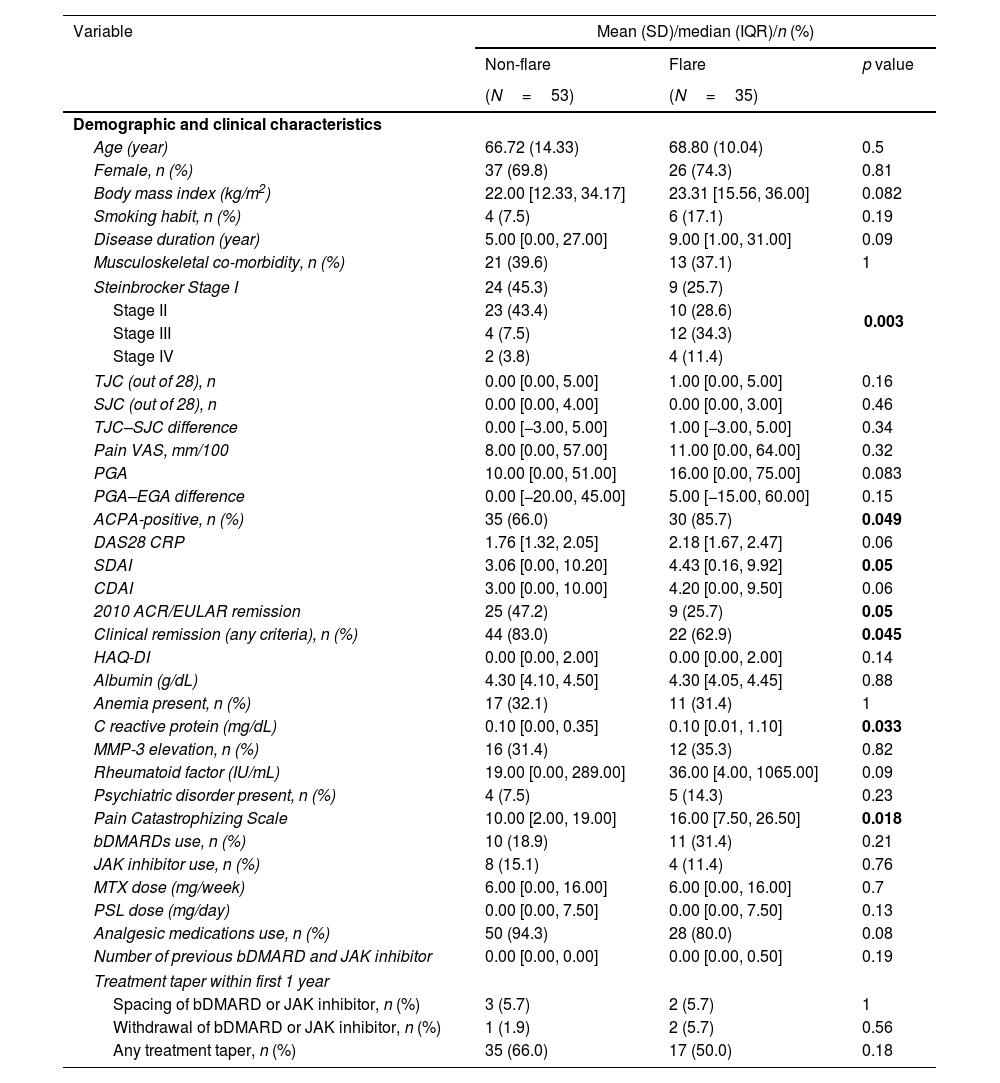
Prediction of the flareThe differences in the demographic and clinical characteristics of the patients between the flare and non-flare groups are shown in Table 3. The prevalence of Steinbrocker stage, ACPA-positivity (p=0.05), 2010 ACR/EULAR remission (p=0.05), and CR according to any criterion (p=0.045) was higher in the flare group. Additionally, DAS28 CRP (p=0.05), SDAI (p=0.05), CRP (p=0.033), and PCS (p=0.018) scores were higher in the flare group. However, no significant differences were found in the HAQ-DI, medication usage, or treatment tapering within 1 year between the flare and non-flare groups. Univariate and multivariate COX regression analyses identified predictors for flares over the 2-year period, as shown in Table 4.
Comparison of the demographic and clinical characteristics between patients who experienced flares and those who did not.
| Variable | Mean (SD)/median (IQR)/n (%) | ||
|---|---|---|---|
| Non-flare | Flare | p value | |
| (N=53) | (N=35) | ||
| Demographic and clinical characteristics | |||
| Age (year) | 66.72 (14.33) | 68.80 (10.04) | 0.5 |
| Female, n (%) | 37 (69.8) | 26 (74.3) | 0.81 |
| Body mass index (kg/m2) | 22.00 [12.33, 34.17] | 23.31 [15.56, 36.00] | 0.082 |
| Smoking habit, n (%) | 4 (7.5) | 6 (17.1) | 0.19 |
| Disease duration (year) | 5.00 [0.00, 27.00] | 9.00 [1.00, 31.00] | 0.09 |
| Musculoskeletal co-morbidity, n (%) | 21 (39.6) | 13 (37.1) | 1 |
| Steinbrocker Stage I | 24 (45.3) | 9 (25.7) | 0.003 |
| Stage II | 23 (43.4) | 10 (28.6) | |
| Stage III | 4 (7.5) | 12 (34.3) | |
| Stage IV | 2 (3.8) | 4 (11.4) | |
| TJC (out of 28), n | 0.00 [0.00, 5.00] | 1.00 [0.00, 5.00] | 0.16 |
| SJC (out of 28), n | 0.00 [0.00, 4.00] | 0.00 [0.00, 3.00] | 0.46 |
| TJC–SJC difference | 0.00 [−3.00, 5.00] | 1.00 [−3.00, 5.00] | 0.34 |
| Pain VAS, mm/100 | 8.00 [0.00, 57.00] | 11.00 [0.00, 64.00] | 0.32 |
| PGA | 10.00 [0.00, 51.00] | 16.00 [0.00, 75.00] | 0.083 |
| PGA–EGA difference | 0.00 [−20.00, 45.00] | 5.00 [−15.00, 60.00] | 0.15 |
| ACPA-positive, n (%) | 35 (66.0) | 30 (85.7) | 0.049 |
| DAS28 CRP | 1.76 [1.32, 2.05] | 2.18 [1.67, 2.47] | 0.06 |
| SDAI | 3.06 [0.00, 10.20] | 4.43 [0.16, 9.92] | 0.05 |
| CDAI | 3.00 [0.00, 10.00] | 4.20 [0.00, 9.50] | 0.06 |
| 2010 ACR/EULAR remission | 25 (47.2) | 9 (25.7) | 0.05 |
| Clinical remission (any criteria), n (%) | 44 (83.0) | 22 (62.9) | 0.045 |
| HAQ-DI | 0.00 [0.00, 2.00] | 0.00 [0.00, 2.00] | 0.14 |
| Albumin (g/dL) | 4.30 [4.10, 4.50] | 4.30 [4.05, 4.45] | 0.88 |
| Anemia present, n (%) | 17 (32.1) | 11 (31.4) | 1 |
| C reactive protein (mg/dL) | 0.10 [0.00, 0.35] | 0.10 [0.01, 1.10] | 0.033 |
| MMP-3 elevation, n (%) | 16 (31.4) | 12 (35.3) | 0.82 |
| Rheumatoid factor (IU/mL) | 19.00 [0.00, 289.00] | 36.00 [4.00, 1065.00] | 0.09 |
| Psychiatric disorder present, n (%) | 4 (7.5) | 5 (14.3) | 0.23 |
| Pain Catastrophizing Scale | 10.00 [2.00, 19.00] | 16.00 [7.50, 26.50] | 0.018 |
| bDMARDs use, n (%) | 10 (18.9) | 11 (31.4) | 0.21 |
| JAK inhibitor use, n (%) | 8 (15.1) | 4 (11.4) | 0.76 |
| MTX dose (mg/week) | 6.00 [0.00, 16.00] | 6.00 [0.00, 16.00] | 0.7 |
| PSL dose (mg/day) | 0.00 [0.00, 7.50] | 0.00 [0.00, 7.50] | 0.13 |
| Analgesic medications use, n (%) | 50 (94.3) | 28 (80.0) | 0.08 |
| Number of previous bDMARD and JAK inhibitor | 0.00 [0.00, 0.00] | 0.00 [0.00, 0.50] | 0.19 |
| Treatment taper within first 1 year | |||
| Spacing of bDMARD or JAK inhibitor, n (%) | 3 (5.7) | 2 (5.7) | 1 |
| Withdrawal of bDMARD or JAK inhibitor, n (%) | 1 (1.9) | 2 (5.7) | 0.56 |
| Any treatment taper, n (%) | 35 (66.0) | 17 (50.0) | 0.18 |
SD: standard deviation; IQR: interquartile range; TJC: tender joints count; SJC: swollen joints count; VAS: Visual Analogue Scale; PGA: patient global assessment; EGA: evaluator's global assessment; ACPA: anti-citrullinated protein/peptide antibody; DAS28: 28-joint count disease activity score; SDAI: simplified disease activity; CDAI: clinical disease activity; ACR: American College of Rheumatology; EULAR: European League Against Rheumatism; HAQ-DI: Health Assessment Questionnaire Disability Index; MMP-3: matrix metalloproteinase-3; bDMARD: biological disease-modifying anti-rheumatic drug; JAK: Janus kinase; MTX: methotrexate; PSL: prednisolone.
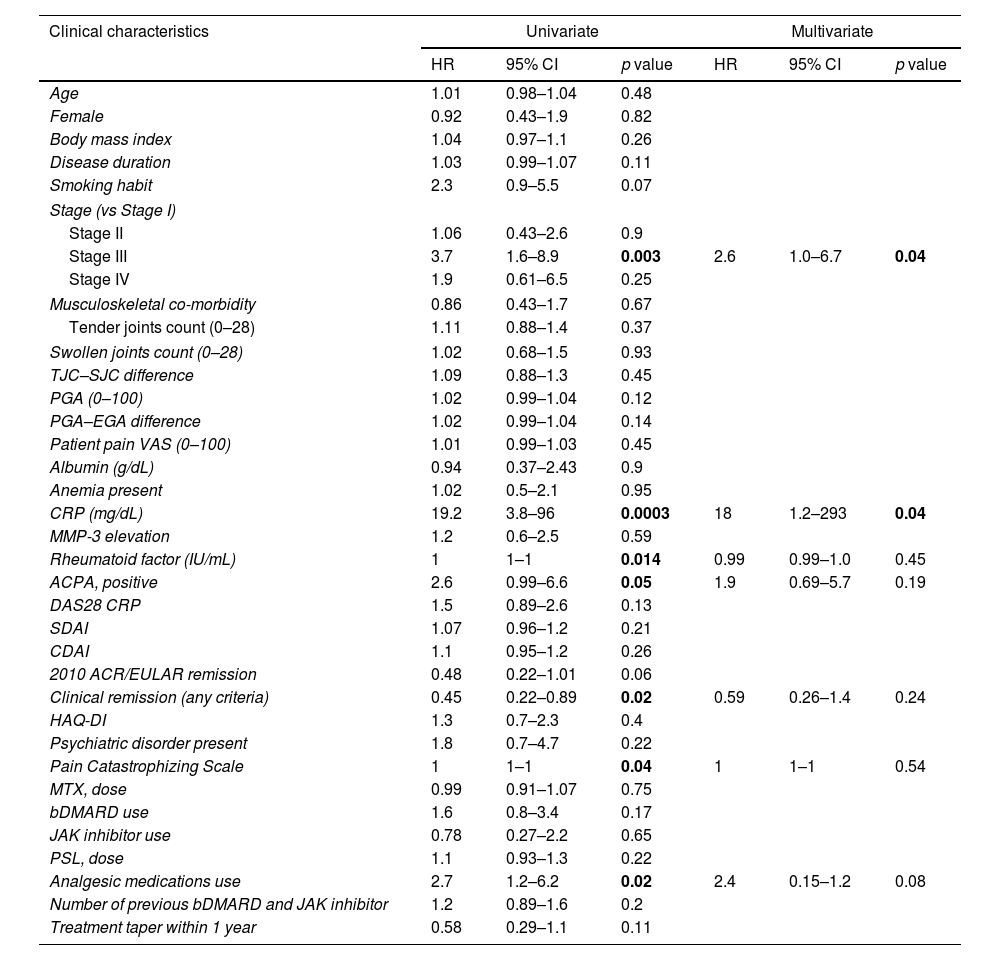
Univariate and multivariate COX regression analysis for predicting flares over 2 years.
| Clinical characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.01 | 0.98–1.04 | 0.48 | |||
| Female | 0.92 | 0.43–1.9 | 0.82 | |||
| Body mass index | 1.04 | 0.97–1.1 | 0.26 | |||
| Disease duration | 1.03 | 0.99–1.07 | 0.11 | |||
| Smoking habit | 2.3 | 0.9–5.5 | 0.07 | |||
| Stage (vs Stage I) | ||||||
| Stage II | 1.06 | 0.43–2.6 | 0.9 | |||
| Stage III | 3.7 | 1.6–8.9 | 0.003 | 2.6 | 1.0–6.7 | 0.04 |
| Stage IV | 1.9 | 0.61–6.5 | 0.25 | |||
| Musculoskeletal co-morbidity | 0.86 | 0.43–1.7 | 0.67 | |||
| Tender joints count (0–28) | 1.11 | 0.88–1.4 | 0.37 | |||
| Swollen joints count (0–28) | 1.02 | 0.68–1.5 | 0.93 | |||
| TJC–SJC difference | 1.09 | 0.88–1.3 | 0.45 | |||
| PGA (0–100) | 1.02 | 0.99–1.04 | 0.12 | |||
| PGA–EGA difference | 1.02 | 0.99–1.04 | 0.14 | |||
| Patient pain VAS (0–100) | 1.01 | 0.99–1.03 | 0.45 | |||
| Albumin (g/dL) | 0.94 | 0.37–2.43 | 0.9 | |||
| Anemia present | 1.02 | 0.5–2.1 | 0.95 | |||
| CRP (mg/dL) | 19.2 | 3.8–96 | 0.0003 | 18 | 1.2–293 | 0.04 |
| MMP-3 elevation | 1.2 | 0.6–2.5 | 0.59 | |||
| Rheumatoid factor (IU/mL) | 1 | 1–1 | 0.014 | 0.99 | 0.99–1.0 | 0.45 |
| ACPA, positive | 2.6 | 0.99–6.6 | 0.05 | 1.9 | 0.69–5.7 | 0.19 |
| DAS28 CRP | 1.5 | 0.89–2.6 | 0.13 | |||
| SDAI | 1.07 | 0.96–1.2 | 0.21 | |||
| CDAI | 1.1 | 0.95–1.2 | 0.26 | |||
| 2010 ACR/EULAR remission | 0.48 | 0.22–1.01 | 0.06 | |||
| Clinical remission (any criteria) | 0.45 | 0.22–0.89 | 0.02 | 0.59 | 0.26–1.4 | 0.24 |
| HAQ-DI | 1.3 | 0.7–2.3 | 0.4 | |||
| Psychiatric disorder present | 1.8 | 0.7–4.7 | 0.22 | |||
| Pain Catastrophizing Scale | 1 | 1–1 | 0.04 | 1 | 1–1 | 0.54 |
| MTX, dose | 0.99 | 0.91–1.07 | 0.75 | |||
| bDMARD use | 1.6 | 0.8–3.4 | 0.17 | |||
| JAK inhibitor use | 0.78 | 0.27–2.2 | 0.65 | |||
| PSL, dose | 1.1 | 0.93–1.3 | 0.22 | |||
| Analgesic medications use | 2.7 | 1.2–6.2 | 0.02 | 2.4 | 0.15–1.2 | 0.08 |
| Number of previous bDMARD and JAK inhibitor | 1.2 | 0.89–1.6 | 0.2 | |||
| Treatment taper within 1 year | 0.58 | 0.29–1.1 | 0.11 | |||
HR: hazard ratio; CI: confidence interval; TJC: tender joints count; SJC: swollen joints count; VAS: Visual Analogue Scale; PGA: patient global assessment; EGA: evaluator's global assessment; ACPA: anti-citrullinated protein/peptide antibody; DAS28: 28-joint count disease activity score; SDAI: simplified disease activity; CDAI: clinical disease activity; ACR: American College of Rheumatology; EULAR: European League Against Rheumatism; HAQ-DI: Health Assessment Questionnaire Disability Index; MMP-3: matrix metalloproteinase-3; bDMARD: biological disease-modifying anti-rheumatic drug; JAK: Janus kinase; MTX: methotrexate; PSL: prednisolone.
In the univariate analysis, significant associations were found between Stage III disease (HR=3.7, 95% CI: 1.6–8.9, p=0.003), CRP levels (HR=19.2, 95% CI: 3.8–96, p=0.0003), RF (HR=1.0, 95% CI: 1.0–1.0, p=0.014), ACPA-positive (HR=2.9, 95% CI: 0.99–6.6, p=0.05), CR (HR=0.45, 95% CI: 0.22–0.89, p=0.02), PCS (HR=1.0, 95% CI: 1.0–1.0, p=0.04), and analgesic medications use (HR=2.7, 95% CI: 1.2–6.2, p=0.02) and a shorter time to flare. In the multivariate analysis, an association between Stage III disease (HR=2.6, 95% CI: 1.0–6.7, p=0.04) and CRP levels (HR=18, 95% CI: 1.2–293, p=0.04) and a shorter time to flare was revealed.
The cutoff value of CRP to predict flares was 0.16mg/dL, with an AUC value of 0.63 (95% CI: 0.512–0.740).
DiscussionThis study yielded three key findings: first, over 2 years of follow-up, 40% of patients experienced a flare, with a mean time to flare of approximately 1 year. Several patients exhibited US-PD flares, as detected by yearly US assessments, highlighting the utility of annual ultrasound evaluations. Conversely, nearly 90% of the patients attained LDA or US remission at both the 1-year and 2-year follow-up visits, indicating a favorable clinical course within this study population. Finally, this study identified Stage III disease and elevated CRP levels as clinical predictors of flares.
The frequency of flares varied depending on the follow-up period, observation period, and flare definitions. In reports of RA patients with LDA or CR, the flare rate was reported to be 30% in a 1-year cohort32 and 34–47% in a 2-year cohort.33,34 In reports on RA patients with both CR and US remission, the flare rate was 39.7%,35 and the time to flare ranged from 9.7 to 30 months.35,36
While these reports are among the few comparable to ours, there are some differences, such as study design (cohort or retrospective), enrollment disease activity criteria (LDA or CR), and different joint sites for US evaluation. For example, Zufferey et al.36 evaluated 22 joint sites, including the bilateral wrist, 1st to 5th MCP, and PIP, elbow, and knee joints. Additionally, differences exist in the definition of US remission, particularly regarding the inclusion or exclusion of tenosynovitis. Regarding the flare rate, our study showed a rate similar to that in previous reports, regardless of whether US remission was present.
Our study showed that the average flare duration was approximately one year, which was shorter than that reported by Zufferey et al.36 Although the flare rate was similar to that in previous studies, the time to flare was shorter than we had assumed, possibly due to the stricter remission state criteria considering the absence of active synovitis and tenosynovitis. The routine use of US imaging may have contributed to these findings. US-PD flares comprised more than half of the flares in our study. Clinically, assessing disease activity in many patients undergoing on-demand US can be challenging, and US assessments may help to detect early synovitis flares. Additionally, some asymptomatic patients have been found to flare on yearly US assessments, suggesting that this assessment method may be important for follow-up.
Although the flare rate was relatively high, the 2-year course of clinical indicators was favorable among patients with LDA and US remission in this study. Even if a flare occurs, disease activity is likely to be well controlled, as shown by the fact that 70% of patients who had a flare within one year return to LDA and US remission in the second year. A potential explanation for the high LDA or CR rates at the 1-year or 2-year visit could be that the patient population was already under tight control and in a state of US remission at the start of the study. Throughout the observation period, tight control was maintained, with frequent use of US monitoring allowing for the early detection of subclinical inflammation. This enabled timely rescue interventions, helping patients achieve LDA or CR even after a flare. Although this hypothesis is speculative, and specific data on the timing and nature of these interventions are not presented in the current results, it is plausible that such tight control, both at baseline and during the study period, contributed to the favorable outcomes. Regarding flare predictors, a previous study identified the duration of CR at baseline using multivariate analysis, which was not considered in our study.35 In our study, Stage III disease and higher CRP levels were modest predictors of flares independent of clinical disease activity measures, seropositivity, psychological factors, and analgesic medication use. This result implies that baseline systemic inflammation according to the CRP level is important in predicting flares, even if the patient is in US remission.
Given that CRP levels are considered to correlate with joint inflammatory activity evaluated by US,37 cases with higher CRP levels in this study should be assumed to have residual synovitis or tenosynovitis at joint sites that were not evaluated. Since all symptomatic joints were evaluated in this study, asymptomatic synovitis or tenosynovitis may have been involved in the flare. Additionally, the sensitivity of PD for detecting synovitis or tenosynovitis should be considered. Indeed, there have been reported cases where pathology revealed residual synovitis despite the absence of synovitis observed on US.38 Thus, the presence of high CRP levels may indicate undetectable residual disease by PD. In contrast, the Steinbrocker stage was a predictor of flares, independent of the CRP level, suggesting that in patients with US remission, joint damage contributes to flares independently of inflammation measures. A previous study on the prediction of flares following remission and treatment withdrawal in early RA reported that MRI measures of bone damage were predictors of disease flares, in addition to inflammatory measures.39 This may indicate that those with RA-specific damage are also at a higher risk of disease flares.
Given this finding, mechanical stress due to joint damage may contribute to disease flares in patients with US remission. One reason why only Stage III disease was revealed as a flare predictor may be the small sample size of patients with Stage IV disease. Another reason may be that Stage IV disease is less likely to cause mechanical stress than Stage III disease due to the narrow range of motion of the joint. We sought to determine whether physical function could be a predictor because the HAQ-DI is a modest predictor of flare-independent baseline PD presence in the patient group with CR.6 However, a previous study on US remission35 did not consider physical function as assessed by the HAQ-DI. In a prior study, the HAQ-DI scores were significantly lower in the US remission group than in the non-US remission group.40 Similarly, the mean HAQ-DI score was low in this study, which may be partly explained by the exclusion of patients with tendonitis or tenosynovitis. We presume that this reduces the effect of physical dysfunction on flares. The Steinbrocker stage, but not the HAQ-DI, emerged as a flare predictor, suggesting that mechanical stress due to joint damage had a stronger influence on flares than physical dysfunction in patients in US remission.
A limitation of our study was the joint region of the US assessment. We assessed only the symptomatic joints, with routine assessments limited to the bilateral hands. Therefore, residual synovitis or tenosynovitis may have been present in the study population. We considered the real-world clinical setting of this study, which aimed to maximize the amount of important joint information while minimizing the time spent performing US assessments.27,28,41 Additionally, the lack of blinding of the US investigators may have introduced bias, particularly affecting pre-test probabilities. This could have impacted the objectivity of the US evaluations and the overall validity of the study's findings. Although this reflects real-world clinical practice, it is a limitation that should be considered when interpreting the results. Additionally, this study did not evaluate synovial hypertrophy using grayscale US (GS), which some reports suggest may be a factor in predicting flares. Some reports suggest that synovial hypertrophy, as defined by GS, may contribute to flares42, indicating that our definition of US remission might be inadequate. Therefore, further investigation into the role of GS in predicting flares of US remission may be warranted. Furthermore, US investigators in our study were not blinded to clinical information in order to maintain consistency with actual clinical operations. This represents a relevant bias in the study that could interfere with the US evaluation. Furthermore, we were unable to completely exclude the presence of false transient PD signals derived from articular overuse, which could affect the accuracy of our findings. Despite these limitations, the strength of the current study is that it is the first US remission study to be performed as a prospective observational cohort, with a long follow-up time involving routine care under daily use of US on-demand and yearly assessments, and the absence of joint synovitis and tenosynovitis.
ConclusionFlares frequently occur in patients with RA who achieve both LDA and US remission. However, most patients in our study showed sustained LDA and US remission at the 1-year and 2-year follow-up visits. Baseline Stage III disease and high CRP levels were modestly predictive of flares in this study. The results of this study may lead to a better assessment of flare risk and enable the selection of personalized treatment strategies for patients with RA who achieve both LDA and US remission.
Ethical approvalThis study was approved by the Kobe Citizen Medical Hospital West's ethics committee (approval number: 19-026) and Hyogo Medical University Hospital's ethics committee (approval number: 3805). All enrolled patients provided written informed consent. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients to be included in the study.
Conflict of interestTakeo Abe, Masao Tamura, Kazuyuki Tsuboi, Yuko Minagawa, Kazuteru Noguchi, Mai Nakano, Chie Ogita, Teppei Hashimoto, Naoto Azuma declare that they have no conflict of interest. Kiyoshi Matsui has received research grants from Asahi-Kasei Pharma and Chugai. This study did not receive any specific grants from funding agencies in the public, commercial, or non-profit sectors.