Coeliac disease (CD) and non-coeliac gluten sensitivity (NCGS) cause symptoms like those seen in patients with fibromyalgia (FM) and functional gastrointestinal disorders. There is no consistent data on frequency of these symptoms and no study performed duodenal biopsies to investigate CD/NCGS in Brazilian FM patients. Therefore, we sought to verify the prevalence of CD/NCGS in FM patients and the association between gastrointestinal manifestations and FM symptoms.
Material and methodsSixty-two individuals with FM (ACR2010) were recruited from FM outpatient clinics of a tertiary hospital. Clinical evaluation included the Widespread Pain Index (WPI), Severity Symptom Scale (SS), Polysymptomatic Distress Scale (PDS), and Fibromyalgia Impact Questionnaire (FIQ). Subjects were screened for the presence of coeliac antibodies and upper gastrointestinal endoscopy (duodenal biopsies) was performed for diagnosis of CD/NCGS.
Results46 (74.2%) women reported at least one digestive symptom: constipation, abdominal distension, loss of weight/inappetence, and nausea/vomiting. Fourteen (31.8%) presented macroscopic duodenitis and 2(4.5%) had duodenal lymphocytic infiltrates, but none met CD criteria. In 1(1.6%) patient NCGS was confirmed. There was association between presence of any digestive symptom and WPI and SS (fatigue, waking up tired, cognition), but no difference on FIQ between patients with and without gastrointestinal symptoms.
ConclusionGastrointestinal complaints were frequent and associated with increased degree of polysymptomatic distress in FM patients, but presence of these symptoms was not related to overall impact of FM over different dimensions of the patient's life. Moreover, the prevalence of CD/NCGS was very low. This suggests that screening for CD in Brazilian FM patients might not be cost-effective, since the frequency of CD/NCGS was very low.
La enfermedad celíaca (EC) y la sensibilidad al gluten no celíaca (SGNC) causan síntomas similares a los observados en pacientes con fibromialgia (FM) y trastornos gastrointestinales funcionales. Ningún estudio realizó biopsias duodenales para investigar EC/SGNC en pacientes brasileños con FM. Por lo tanto, buscamos verificar la prevalencia de EC/SGNC en pacientes con FM y la asociación entre manifestaciones gastrointestinales y síntomas de FM.
Material y métodosSesenta y dos mujeres con FM (ACR2010) fueron reclutadas de las consultas de FM de un hospital terciario. La evaluación incluyó el índice de dolor generalizado (IDG), la escala de gravedad de síntomas (SS), la escala de angustia polisintomática (EAP) y el cuestionario de impacto de la fibromialgia (FIQ). Los sujetos fueron examinados para la presencia de anticuerpos celíacos y se realizó una endoscopia gastrointestinal superior (biopsias duodenales) para el diagnóstico de EC/SGNC. Se investigaron las asociaciones estadísticas entre las molestias gastrointestinales y los síntomas de FM (p<0,05).
ResultadosUn total de 46 (74,2%) mujeres refirieron al menos un síntoma digestivo: estreñimiento, distensión abdominal, pérdida de peso/inapetencia y náuseas/vómitos. Catorce (31,8%) presentaban duodenitis macroscópica y 2 (4,5%) infiltrados linfocíticos duodenales, pero ninguno cumplía criterios de EC. En un (1,6%) paciente se confirmó SGNC. Hubo asociación entre la presencia de síntoma digestivo y IDG y SS (fatiga, despertarse cansado, cognición), pero no hubo diferencia en FIQ entre pacientes con y sin síntomas gastrointestinales.
ConclusiónA pesar de la alta prevalencia de síntomas digestivos y su asociación con el grado de amplificación del dolor central, la frecuencia de EC/SGNC fue insignificante. Además, no se observaron diferencias en el impacto de la FM en la calidad de vida (FIQ) en pacientes con y sin síntomas gastrointestinales.
Fibromyalgia (FM) is a specific disorder characterized by chronic widespread pain that belongs to the central sensitization syndromes spectrum.1 In addition to pain, chronic fatigue and sleep disturbances take part of the core symptoms of the disease.2 Up to 50–70% of patients also exhibit nonspecific gastrointestinal manifestations such as diarrhea, constipation, nausea, dyspepsia, and abdominal distention.2,3 Despite being very frequent, these symptoms are commonly overlooked by doctors and do not receive adequate attention. Frequently these manifestations are referred to as “irritable bowel syndrome (IBS)”.4,5
Recently, some authors have suggested a connection between gluten sensitivity disorders and FM. Indeed, most gastrointestinal symptoms exhibited by patients with FM are also found in patients with celiac disease or gluten sensitivity.6–8 Moreover, there is some evidence that gluten-free diet in this subgroup of patients can produce a slight but significant improvement in FM symptoms.8
Celiac disease (CD) is a chronic autoimmune disorder, primarily affecting the small intestine, caused by a reaction to dietary gluten. CD may manifest without gastrointestinal symptoms; in fact, nearly half of the CD patients diagnosed in adulthood do not have relevant gastrointestinal manifestations. In contrast, they presented with asthenia, arthralgias and myalgias, mental fatigue, insomnia, and depression, which are symptoms that also occurred in nociplastic pain syndromes such as fibromyalgia.9
Non-celiac gluten sensitivity (NCGS) is an emerging entity characterized by gluten-related intestinal and extraintestinal symptoms in patients with negative CD tests. Thus, they do not meet criteria for CD, although their symptoms improve on a gluten-free diet.10,11 Recently, evidence has showed that this entity is even more prevalent than CD, which affects 1% of the population.12 Although it may be associated to intraepithelial lymphocytosis injury to the intestinal, it neither provokes antibody production nor atrophy of the intestinal villi epithelium. Therefore, unlike celiac disease, gluten sensitivity disorder is not an autoimmune disease.12
There is no consistent data on frequency of gastrointestinal symptoms as well as no study performed duodenal biopsies to investigate CD/NCGS in Brazilian FM patients. Thus, we sought to investigate the frequency of CD and NCGS in Brazilian patients with FM treated at the outpatient clinic of a tertiary hospital, as well as to verify the association between gastrointestinal manifestations and classic FM symptoms and their impact on patients’ quality of life.
Material and methodsBetween June 2016 and April 2017, 62 FM patients were recruited from the outpatient FM of a single center, Rheumatology Division, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo – HCFMUSP, São Paulo, Brazil. All patients fulfilled the American College of Rheumatology 2016 diagnostic criteria for fibromyalgia.2 Exclusion criteria were patient refusal, previous diagnosis of autoimmune rheumatic disease, inflammatory bowel disease, chronic hepatitis C, vitamin deficiencies, immunodeficiencies, other food intolerances or chronic diseases with inadequate control, such as diabetes and hypo-/hyperthyroidism.
This study was approved by the Local Ethics Committee, and all participants gave written informed consent.
Clinical variablesClinical information was obtained by a standardized questionnaire administered in person. The form included questions on age, sex, race, comorbidities, current medication(s) and relevant gastrointestinal symptoms, including those characteristics of celiac disease: diarrhea, constipation, abdominal pain, bloating, weight loss, inappetence, nausea/vomiting.
Height, weight, and body mass index (BMI) of each participant were measured using standard protocols. BMI was calculated by dividing the participants’ weight (kg) by their height squared (m2).
To evaluate the overall impact of FM over different dimensions of the patient's life, we used the numerical indices which are part of the diagnostic criteria: Widespread Pain Index (WPI), Symptoms Severity (SS) and Polysymptomatic Distress Scale (PDS).2,13 Also, the validated Brazilian version of the Fibromyalgia Impact Questionnaire (FIQ) was also used. The FIQ comprises a 10-item scale that range between 0 and 100 points, derived from various questions about physical functioning, work status level, degrees of depression and anxiety, sleep alterations, severity of pain, stiffness, fatigue and the perception of wellbeing. Total FIQ scores were divided into three categories, ranging from 0 to 39 (mild), 40 to 59 (moderate), and 60 and over (severe).14,15
Laboratory variablesAnti-IgA tissue transglutaminase (tTG) antibodies were measured with enzyme-linked immunosorbent assays (ELISA) to detect IgA anti-guinea pig liver (Product No. T-5398. Sigma–Aldrich Co., St. Louis, MO, USA). IgA anti-endomysial antibodies (EMA) were verified by immunofluorescence test using human umbilical cord, which was incubated with goat anti-human fluorescein-conjugated IgA (Product No. F-9637, Sigma–Aldrich Co., St. Louis, MO, USA).
Other exams, including blood count, albumin, calcium (adjusted for the albumin concentration), phosphorus, creatinine, TSH, glucose, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were determined using standard automated laboratory methods.
Upper gastrointestinal endoscopy and small bowel biopsyAn upper gastrointestinal endoscopy with at least four duodenal biopsies was performed and samples were studied for the presence and count the number of intraepithelial lymphocytes (IELs) throughout hematoxylin and eosin (H&E) stain and anti-CD3 immunohistochemical monoclonal antibodies. Samples were analyzed by two expert pathologists and classified according to the histological classification of CD described by Marsh-Oberhüber16,17: stage 0, normal duodenum; stage 1, increased IEL infiltration with a total count of ≥25%; stage 2, crypt hyperplasia and presence of diffuse chronic inflammatory infiltration of the lamina propria; stage 3, villous atrophy.
CD was defined by the presence of IEL>40/100 enterocytes and Marsh-Oberhüber 2 or 3 plus positive anti-tTG or EmA. NCGS was diagnosed when IEL>25/100 enterocytes, no vilous atrophy (Marsh-Oberhüber 0, 1 or 2) and negative antibodies plus presence of symptoms (nausea, diarrhea, constipation, bloating and flatulence).18–20
Statistical analysisContinuous variables were expressed as means±standard deviations (SDs) and categorical variables as counts and percentages. PDS was also analyzed as tertiles. The chi-square test, Fisher's test, likelihood ratio test, unpaired Student's t test or Mann–Whitney's test was used to verify the association between gastrointestinal symptoms and classical manifestations of fibromyalgia. Significance was set at p<0.05. All analyses were performed using SPSS software version 21.0 for windows.
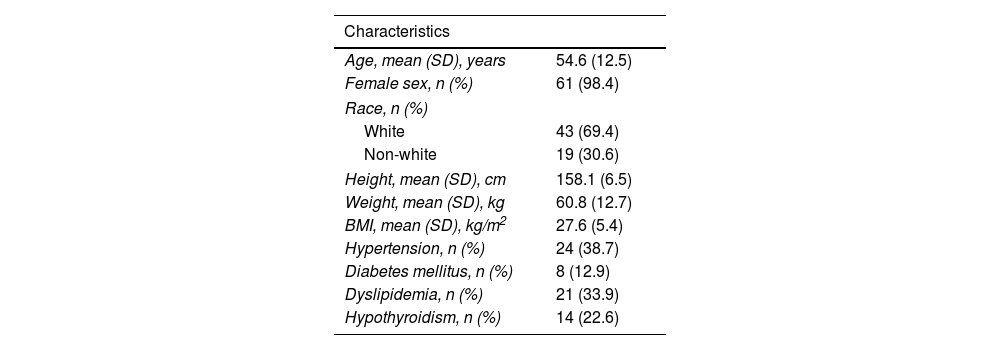
ResultsDemographic and clinical characteristics of the patients are shown in Table 1. Ninety-eight per cent of patients were women. Mean age of the complete sample was 54.6±12.5 years.
Clinical features of the subjects.
| Characteristics | |
|---|---|
| Age, mean (SD), years | 54.6 (12.5) |
| Female sex, n (%) | 61 (98.4) |
| Race, n (%) | |
| White | 43 (69.4) |
| Non-white | 19 (30.6) |
| Height, mean (SD), cm | 158.1 (6.5) |
| Weight, mean (SD), kg | 60.8 (12.7) |
| BMI, mean (SD), kg/m2 | 27.6 (5.4) |
| Hypertension, n (%) | 24 (38.7) |
| Diabetes mellitus, n (%) | 8 (12.9) |
| Dyslipidemia, n (%) | 21 (33.9) |
| Hypothyroidism, n (%) | 14 (22.6) |
BMI: body mass index.
Any gastrointestinal symptom was observed in 46 (74.2%) individuals. The prevalence of the symptoms, in descending order of frequency, were bloating (35, 56.5%), constipation (34, 54.8%), weight loss/inappetence (21, 33.9%), nausea/vomiting (21, 33.9%) and diarrhea (9, 14.5%). The mean values of the FM indexes were: WPI 8.6±4.5; SS 8.6±2.6; PDS 16.9±6.0 and FIQ 56.5±14.2.
Concerning to antibodies related to celiac disorder, no patient had positivity for tTG or EMA. Of 62 patients, 44 (71%) underwent upper digestive endoscopy. The anatomopathological findings of endoscopy were esophagitis in 10 individuals (22.7%), gastritis in 22 (50%) and duodenitis in 14 (31.8%). Only 2 (%) patients had intraepithelial limphocytosis>25%. No patient had celiac disease, according to Marsh-Oberhüber criteria. Diagnosis of NCGS was made in only one subject (2.3%).
Compared to those with no gastrointestinal symptoms, patients with at least one gastrointestinal manifestation had similar mean values of SS (8.9±2.3 vs. 7.9±3.1, p=0.318), PDS (17.2±5.7 vs 17.7±6.4, p=0.871) and FIQ (58.0±13.5 vs 52.1±15.7, p=0.128). Mean number of painful areas (WPI) were greater in people with gastrointestinal symptoms (9.3±4.6 vs. 6.6±3.9, p=0.027).
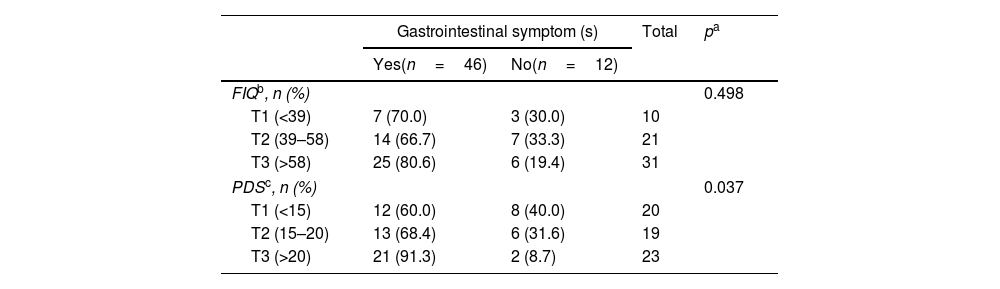
Although there was no significant association between presence of gastrointestinal symptoms and the mean value of PDS, when we categorize the scale in tertiles, those participants in the highest tertile (T3: >20) reported more frequently the presence of gastrointestinal symptoms (91.3%), compared with subjects in the lowest tertile (T1: <15) (8.7%) (p=0.037) (Table 2).
FIQ and PDS scales according to presence of gastrointestinal symptoms.
In female patients from a Brazilian tertiary hospital, we did not find any case of CD and only woman had NCGS. Nevertheless, almost 75% of the subjects had gastrointestinal symptoms (more than half referring constipation or abdominal distension). Moreover, a positive association between these manifestations and greater central pain amplification was observed.
The study has some strengths, such as the inclusion of a homogeneous sample of patients with long-standing FM from a tertiary hospital and exclusion of patients with conditions that could cause gastrointestinal disturbances. Furthermore, diagnosis of CD/NCGS was accurate since we carried out duodenal biopsy to verify the presence of lymphocytic enteritis. Several prevalence surveys on gastrointestinal disorders in FM did not perform histopathological diagnosis.5,6
In recent years, some evidence has been showed that CD and NCGS have a high prevalence in FM patients,6–8 different from our findings. In a Spanish study with 104 FM patients with associated IBS, the frequency of CD was 6.7%, and 56% of lymphocytic enteritis (Marsh type 1 lesion) was observed in duodenal biopsies.7 Moreover, in CD patients from United States, 9% of them had FM, which is higher than the prevalence of FM in general population.21 In Italy, prevalence of CD in FM was 1%,22 quite similar to our results. These discrepancies may be explained by the fact that the worldwide prevalence of CD varies according to the geographical localization and genetic background of the population. It is more common in people bearing HLA DQ2 and HLA DQ8.23 In Brazil, a previous survey in 94 FM patients found that only one (1.1%) had positive IgG anti-tTG test. This positive subject was submitted to gastrointestinal endoscopy with small bowel biopsy and result was histologically normal (relationship villus-crypt of 3:1 and with 15lymphocytes/100 cells).24 Herein, we observed only one patient with NCGS (2.3%) e none with CD.
Our study confirms and extends findings reported in other populations that also have high prevalence of gastrointestinal complaints in FM patients. Triadafilopoulos et al. showed similar frequency of gastrointestinal problems in patients with FM (73%).25 In a Spanish survey, patients with FM reported gastrointestinal symptoms more often than controls (98% vs 49%).5
Digestive symptoms in patients with FM4 are related to changes in the autonomic nervous system and functional disorders, as well as psychological stress factors. Furthermore, the overlap of FM with some central sensitivity conditions such as irritable bowel syndrome (IBS), migraines, chronic fatigue syndrome, and mood disorders, suggest common etiologic factors.3,4
Furthermore, we demonstrated that individuals with digestive complaints reported an increased intensity cardinal symptom of FM, such as painful areas, fatigue, unrefreshed sleep and dyscognition. Despite this, these individuals did not have worse FIQ values compared to patients without digestive symptoms, suggesting that their presence does not necessarily translate into a greater impairment in patient's life.
This study has some limitations, such as the limited size of the sample. Moreover, 29% of the subjects did not undergo endoscopy and biopsy, which can underestimate the true prevalence of disease related to gluten sensitivity in the population studied. Also, the absence of a healthy control group did not allow us extrapolate study findings to the general population. However, it is well established that FM patients have higher frequency of digestive symptoms, and we sought primarily to assess the association between gastrointestinal complaints and main FM domains. Thus, ultimately, the absence of a control group does not hamper the interpretation of the results related to the primary aim of the study. In addition, patients were selected exclusively from a tertiary hospital and this sample might not be representative of the overall Brazilian FM population. Another limitation comes from potential selection bias since subjects who agree to participate in a study could be more likely to communicate symptoms of any nature and this factor could overestimate the results.
In conclusion, we reported not only a high prevalence of gastrointestinal symptoms in FM patients, but also an association between these manifestations and increased degree of polysymptomatic distress (fatigue, unrefreshed sleep, and cognitive problems). On the other hand, occurrence of gastrointestinal complains was not related to overall impact of FM over different dimensions of the patient's life. Moreover, our results suggest that searching for CD in Brazilian FM patients might not be cost-effective since the frequency of CD/NCGS was very low.
Funding sourceThis study was not sponsored by any pharmaceutical companies.
Conflict of interestZanetti CB has no disclosures. Pontes MAG has no disclosures. Moura EGH has no disclosures. Domiciano DS has no disclosures.