To determine the incidence and prevalence of cancer in a cohort of patients with systemic lupus erythematosus (SLE) and identify associated risk factors.
Patients and methodsThe study comprised a dynamic cohort of SLE patients (November 1989 to December 2006) at a tertiary referral centre. An adjusted external control from the hospital tumour registry was used.
ResultsThe study included 175 SLE patients (90% women), with a mean time at risk of 1370.5 patient-years. Fourteen women (8%) died, mainly from cardiovascular events. No patient died due to malignancy. We found 35 tumours in 28 patients, 25 of them after the diagnosis of SLE, of which 5 were malignant. The rate of benign tumours was 14.6/1000 patient-years (95% CI, 8.9–22.5) and of malignant tumours 3.6/1000 patient-years (95% CI, 1.5–8.8), with a crude incidence odds ratio for malignant tumours of 3.5 (95% CI, 1.5–7.9). However, this significance was lost after standardizing the rates. Concerning associated risk factors, differences were found in the mean erythrocyte sedimentation rate [HR 1.4 (1.1–1.7)], and the presence of thrombosis [HR 6.9 (1.49–41.2)], especially arterial thrombosis.
ConclusionsWe found a crude incidence rate of cancer that was almost four times greater in our SLE patients as compared with the expected rate in the hospital area of western Malaga.
Determinar la incidencia y prevalencia del cáncer en una cohorte de pacientes con lupus eritematoso sistémico (LES) e identificar los factores de riesgo asociados.
Pacientes y métodosEl estudio incluyó una cohorte dinámica de los pacientes con LES (de noviembre de 1989 a diciembre del 2006) en un centro hospitalario de tercer nivel. Se utilizó un control externo ajustado por edad y sexo a través de un registro hospitalario de tumores de la misma área sanitaria.
ResultadosEl estudio incluyó a 175 pacientes con LES (90% mujeres), con un tiempo en riesgo de 1370,5 pacientes-año. Catorce mujeres (8%) murieron, principalmente por eventos cardiovasculares. Ningún paciente falleció por tumor maligno. Se encontraron 35 tumores en 28 pacientes, 25 de ellos después del diagnóstico de LES, de los cuales 5 fueron malignos. La tasa de tumores benignos fue de 14,6/1000 pacientes-año (IC del 95%, 8,9–22,5) y de los tumores malignos 3,6/1000 pacientes-año (IC del 95%, 1,5 a 8,8), con una razón de momios de incidencia cruda para los tumores malignos de 3,5 (IC del 95%, 01,05 a 07,09). Sin embargo, esta significación se perdió cuando se estandarizaron las tasas. En cuanto a los factores de riesgo asociados, se encontraron diferencias en la velocidad de sedimentación globular media (HR 1,4 [1,1–1,7]), y la presencia de trombosis (HR 6,9 [1,49 a 41,2]), en especial la trombosis arterial.
ConclusionesEncontramos una tasa cruda de incidencia de cáncer casi 4 veces mayor en los pacientes con LES en comparación con la tasa esperada en nuestra área de influencia del hospital (zona oeste de Málaga).
Systemic lupus erythematosus (SLE) is an inflammatory disease mediated by immunological mechanisms with a complex genetic basis. Interaction between genes and environmental factors favouring this disease have resulted in a progressively more self-aggressive immune system with a greater capacity for harming the body's own organs and systems. This complex gene-environment interaction is associated with a diverse phenotype expression and comorbid consequences that shorten life expectancy and quality of life in SLE patients. Although the accelerated atherosclerosis experienced by patients with lupus and other inflammatory disorders is one of the late consequences that has received most attention, neoplasms have also received the attention of many researchers. However, the results of research on this latter aspect are not entirely homogenous.1,2 This disparity is probably due in part to the methods used in the various studies, as well as to the very essence of the mechanisms involved in the development of cancer and SLE. For instance, we know that genes involved in the development of lupus vary between one population and another, as too does the incidence of cancer between different geographical areas, mainly due to environmental factors, though genetic factors also play a part.3,4 In Spain this aspect is poorly known and has never been examined with controlled studies. The aim, therefore, of this study was to determine the incidence of cancer in a cohort of SLE patients from southern Spain and compare the result with a similar population without SLE.
Patients and MethodsThis prospective, controlled dynamic cohort study was undertaken between February 1990 and December 2006 in a population over 14 years of age seen at the Hospital Universitario Virgen de la Victoria in Malaga, Spain. This tertiary referral hospital for cancer and autoimmune diseases covers 475,017 inhabitants from the western part of the province of Malaga. The SLE cohort was hospital based and started in 1990. Data from a few patients treated in our unit until then were collected retrospectively. However, the follow-up was fully prospective since 1990 and an established protocol for the collection of data and biological samples was used. Patients have been followed up in outpatient clinics and in the hospital as inpatients. The study was approved by the hospital ethics and research committee.
CasesThe study included 175 patients with SLE classified according to the revised criteria of the American College of Rheumatology (ACR) for SLE.5 To be included as incident cases of cancer the patients had to fulfil the norms of the World Health Organization (WHO)6 incorporated in the ICD-O (International Classification of Diseases for Oncology). The cases of cancer were confirmed histologically and obtained from the hospital tumour registry.
ControlsThe control group was obtained from the hospital tumour registry of the Hospital Universitario Virgen de la Victoria. This active registry, compiled by the Pathology Department of Malaga University, at the School of Medicine, keeps a continuous, systematic record, with data taken from the Pathology Department, Clinical Records, Oncology and Haematology Services, concerning all cases of malignant tumours (both carcinomas in situ and malignant) diagnosed and/or treated since 1 January 1993 at any of the hospital services. For the patients to be included, the incident cases had to follow the WHO norms,6 indicated in the ICD-O. This registry forms part of the National Network of Hospital Tumour Registries, the aim of which is merely descriptive as it holds no data on tumour incidence.
Study Protocol and VariablesThe variables were collected prospectively and included in an electronic database when the patients entered our SLE cohort. The data were updated at each hospital visit, which varied in frequency according to the clinical needs of the patients, though the maximum time between visits was 6 months.
Information recorded for all the SLE patients included personal data, risk factors, such as smoking habit (non-smokers, ever-smokers or active smoker) and alcohol consumption), body mass index (BMI) (not obese: BMI<29.9kg/m2, and obese: BMI>30), hypertension,7 diabetes mellitus,8 dyslipidemia,8 and gynaecological history.
Data relating to SLE included date of onset of SLE, defined by the presence of signs or symptoms suggesting lupus; date of diagnosis of SLE, defined as the first date on which the patient objectively fulfilled 4 or more of the ACR criteria; the SLE symptoms present at inclusion to the cohort and on each visit (recorded prospectively); the disease activity at the time of the protocol, using SLEDAI-2K9 and SLICC/ACR DI10; disease severity11; and treatment used during the disease, including immunosuppressant drugs. The registry recorded data from patients from the same reference area as the cases. The data from the tumour registry were cross-matched with those in our SLE database to confirm that no SLE patient was counted as a control.
Tumour-related information included the data of incidence of cancer, defined by the histological diagnosis of the cancer, tumour type, number of tumours the patient had, histological tumour type, state at diagnosis, treatment and result, i.e., remission, progression or death. Tumours diagnosed before and after the diagnosis of SLE were included, though for the statistical analysis we only considered those tumours diagnosed after the diagnosis of SLE. The date of inclusion in the cohort was defined as the date on which the patient started follow-up in our hospital.
Statistical AnalysisTo estimate the risk of cancer and compare it between populations, respective calculations were made of the incidence rates and the standardized incidence ratios (SIR). The 95% confidence intervals (CI) were calculated using the Poisson method.12 The incidence rate of cancer in the SLE cohort was calculated by dividing the number of cancers detected by the “time at risk” of the cohort. The “time at risk” was calculated in person-years, adding the times that each SLE patient had remained under observation in the cohort, from the date of diagnosis of SLE to the date of the first cancer (incident case), loss to follow-up, death or end date of the observation period (31 December 2006 – censored case). To calculate the incidence rates we excluded cases of cancer diagnosed prior to the diagnosis of SLE. The SIR was adjusted for age and sex by the indirect method or internal standardization. The number of expected cases was obtained from the incidence rate of the registry, adjusted for age, sex and calendar year of the cases. The incidence rate of cancer in the registry was calculated by dividing the number of cancers detected by the “time at risk” of the inhabitants covered by the tertiary referral hospital between February 1990 and December 2006. The “time at risk” was calculated in person-years taking into account the annual variations for men and women in our population census registries during the same period.
Normality was assessed by the Kolmogorov–Smirnov test. Contrast of the quantitative variables was done with the Student t test for independent variables or the Wilcoxon-Mann–Whitney U test, and for the qualitative variables using the Pearson χ2-test, with the Fisher exact test if necessary. The significance shown beside the SIR in each table was obtained with the Mantel–Haenszel homogeneity test (M–H).13 The multivariate analysis of the factors associated with the incidence of cancer in the SLE cohort was done using Cox regression, considering those patients without cancer at the end of the follow-up or death as censored cases. The results are expressed as the hazards ratio (HR) with their 95% CI. The multivariate analysis only included those variables that reached P<.1 in the univariate analysis. The erythrocyte sedimentation rate (ESR) values introduced into the model correspond to the mean values observed during the follow-up. The multivariate model was adjusted for age using the Wald forward stepwise method with an input likelihood of 0.05 and an output likelihood of 0.1. The results computed were both crude and age-adjusted. All the calculations and the statistical analyses were done with SPSS 14.0 and the epidemiological analysis with STATA 10.0
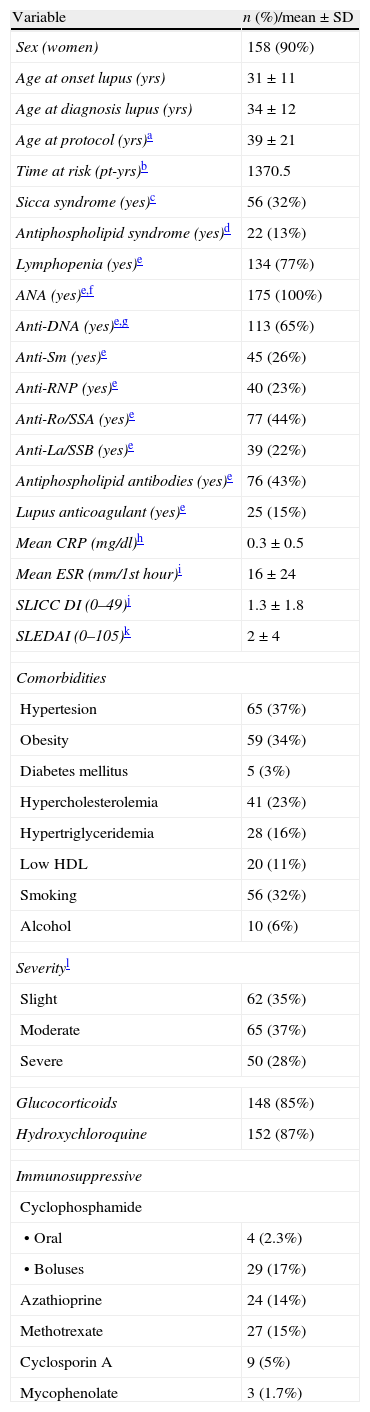
ResultsThe cohort comprised 175 SLE patients included between November 1989 and December 2006. Table 1 summarizes the descriptive data of the cohort. Most patients were younger than 40 years of age at the end of the follow-up and there was no male patient aged over 65 years. Proportionally more men than women abandoned the follow-up [7 (40%) vs. 22 (14%); P=.018].
Demographics, Clinical Factors, and Exposure Histories of SLE Patients.
| Variable | n (%)/mean±SD |
| Sex (women) | 158 (90%) |
| Age at onset lupus (yrs) | 31±11 |
| Age at diagnosis lupus (yrs) | 34±12 |
| Age at protocol (yrs)a | 39±21 |
| Time at risk (pt-yrs)b | 1370.5 |
| Sicca syndrome (yes)c | 56 (32%) |
| Antiphospholipid syndrome (yes)d | 22 (13%) |
| Lymphopenia (yes)e | 134 (77%) |
| ANA (yes)e,f | 175 (100%) |
| Anti-DNA (yes)e,g | 113 (65%) |
| Anti-Sm (yes)e | 45 (26%) |
| Anti-RNP (yes)e | 40 (23%) |
| Anti-Ro/SSA (yes)e | 77 (44%) |
| Anti-La/SSB (yes)e | 39 (22%) |
| Antiphospholipid antibodies (yes)e | 76 (43%) |
| Lupus anticoagulant (yes)e | 25 (15%) |
| Mean CRP (mg/dl)h | 0.3±0.5 |
| Mean ESR (mm/1st hour)i | 16±24 |
| SLICC DI (0–49)j | 1.3±1.8 |
| SLEDAI (0–105)k | 2±4 |
| Comorbidities | |
| Hypertesion | 65 (37%) |
| Obesity | 59 (34%) |
| Diabetes mellitus | 5 (3%) |
| Hypercholesterolemia | 41 (23%) |
| Hypertriglyceridemia | 28 (16%) |
| Low HDL | 20 (11%) |
| Smoking | 56 (32%) |
| Alcohol | 10 (6%) |
| Severityl | |
| Slight | 62 (35%) |
| Moderate | 65 (37%) |
| Severe | 50 (28%) |
| Glucocorticoids | 148 (85%) |
| Hydroxychloroquine | 152 (87%) |
| Immunosuppressive | |
| Cyclophosphamide | |
| • Oral | 4 (2.3%) |
| • Boluses | 29 (17%) |
| Azathioprine | 24 (14%) |
| Methotrexate | 27 (15%) |
| Cyclosporin A | 9 (5%) |
| Mycophenolate | 3 (1.7%) |
The “time at risk” was calculated adding the times that each SLE patient had remained under observation in the cohort, from the date of diagnosis of SLE to the date of the first cancer (incident case), lost to follow-up, death or end date of the observation period (31 December 2006 – censored case).
APS was classified according to the Sydney revision of the Sapporo criteria (Miyakis et al., J Thromb Haemost 2006, 4:295–306).
Slight, when, either in the past or at present, important organs such as the kidneys, central nervous system, heart or lungs, had been not affected; Moderate, when only one of these organs had been o was affected; and Severe, when two or more of these organs had been affected or aggressive therapy had been required for the complications, such as more than 50mg of prednisone daily, pulse therapy with steroids or immunosuppressant drugs.
Concerning immunosuppressive therapy, 96 patients (55%) received at some time at least one immunosuppressive drug, mostly bolus cyclophosphamide, followed by methotrexate and azathioprine.
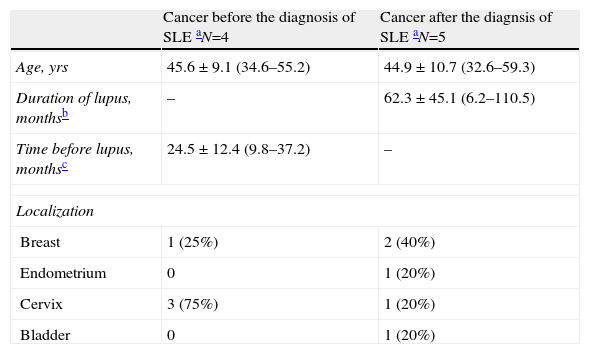
Frequency and Types of CancerIn the whole SLE cohort, nine patients had cancer, four of whom had cancer before diagnosis of SLE and five after diagnosis of SLE. Table 2 shows the characteristics of the cancers. The mean age at diagnosis of the cancer in those who already had SLE was 45 years, with a mean of five years between diagnosis of SLE and diagnosis of the cancer. The most usual type of cancer before SLE was cancer of the cervix, followed by breast cancer. However, in those whose cancer was diagnosed after their diagnosis of SLE, the most common type was breast cancer. Most cancers were diagnosed in situ or at stage I (6 of 9) and all were treated surgically; only one patient, with breast cancer, also required chemotherapy and radiotherapy. All recovered satisfactorily. One of the cervical cancers was stage IIIb, and the patient was treated successfully with surgery and radiotherapy. Only two cancers recurred, one stage IV breast cancer and one bladder cancer (PT1-AG1). No malignant haematological neoplasm was detected.
Descriptive Characteristics of SLE Patients With Cancer.
| Cancer before the diagnosis of SLE aN=4 | Cancer after the diagnsis of SLE aN=5 | |
| Age, yrs | 45.6±9.1 (34.6–55.2) | 44.9±10.7 (32.6–59.3) |
| Duration of lupus, monthsb | – | 62.3±45.1 (6.2–110.5) |
| Time before lupus, monthsc | 24.5±12.4 (9.8–37.2) | – |
| Localization | ||
| Breast | 1 (25%) | 2 (40%) |
| Endometrium | 0 | 1 (20%) |
| Cervix | 3 (75%) | 1 (20%) |
| Bladder | 0 | 1 (20%) |
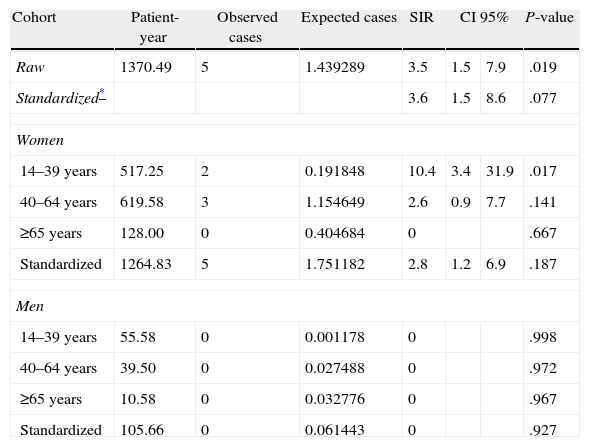
Table 3 shows that the crude incidence rate of cancer after diagnosis of SLE was 3.6 per 1000 patient-years (95% CI, 1.5–8.8). Our hospital tumour registry, used to calculate the expected cases, recorded 2654 cancers in 2,527,047 patient-years, giving a crude incidence rate of expected cases of 1.05 per 1000 person-years (95% CI, 1.01–1.09). The same table also shows that the stratum-specific odds ratios of the incidence rates differed, and the effect of the presence of SLE may not be the same in all age groups, as it seems to decline with older age.
Incidence Rates Cancer in 175 Patients After Diagnosis of Lupus.
| Cohort | Patient-year | Observed cases | Expected cases | SIR | CI 95% | P-value | |
| Raw | 1370.49 | 5 | 1.439289 | 3.5 | 1.5 | 7.9 | .019 |
| Standardized* | 3.6 | 1.5 | 8.6 | .077 | |||
| Women | |||||||
| 14–39 years | 517.25 | 2 | 0.191848 | 10.4 | 3.4 | 31.9 | .017 |
| 40–64 years | 619.58 | 3 | 1.154649 | 2.6 | 0.9 | 7.7 | .141 |
| ≥65 years | 128.00 | 0 | 0.404684 | 0 | .667 | ||
| Standardized | 1264.83 | 5 | 1.751182 | 2.8 | 1.2 | 6.9 | .187 |
| Men | |||||||
| 14–39 years | 55.58 | 0 | 0.001178 | 0 | .998 | ||
| 40–64 years | 39.50 | 0 | 0.027488 | 0 | .972 | ||
| ≥65 years | 10.58 | 0 | 0.032776 | 0 | .967 | ||
| Standardized | 105.66 | 0 | 0.061443 | 0 | .927 | ||
SIR, standardized incidence ratios; CI, confidence intervals.
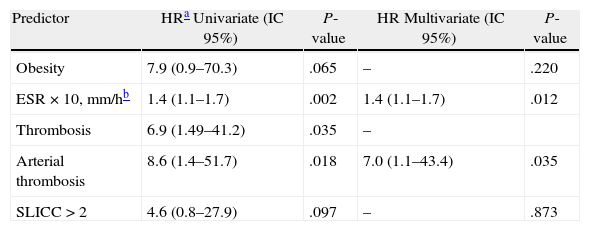
As it can be seen in Table 4, of the variables included in the regression analyses, only history of thrombosis [HR 6.9 (95% CI, 1.49–41.2; P=.035], especially of the arterial territory [HR 8.6 (95% CI, 1.4–51.7); P=.018] and mean ESR [HR for each 10mm/h 1.4 (95% CI, 1.1–1.7); P=.002] showed a significant association with the incidence of cancer. Nonetheless, both obesity and a SLICC score above 2 also showed a certain association. In multivariate analysis, only ESR [HR for each 10mm/h 1.4 (95% CI, 1.1–1.7); P=.012] and arterial thrombosis [HR 7.0 (95% CI, 1.1–43.4); P=.035] remained associated. We found no association between the incidence of cancer and other manifestations of SLE, nor with alcohol consumption, smoking, age at diagnosis, oral contraceptives, drugs used for the SLE, autoantibodies, complement consumption or disease severity.
Uni- and Multivariate Cox Regression Analysis of Risk Factors for Cancer in SLE Patients.
| Predictor | HRa Univariate (IC 95%) | P-value | HR Multivariate (IC 95%) | P-value |
| Obesity | 7.9 (0.9–70.3) | .065 | – | .220 |
| ESR×10, mm/hb | 1.4 (1.1–1.7) | .002 | 1.4 (1.1–1.7) | .012 |
| Thrombosis | 6.9 (1.49–41.2) | .035 | – | |
| Arterial thrombosis | 8.6 (1.4–51.7) | .018 | 7.0 (1.1–43.4) | .035 |
| SLICC>2 | 4.6 (0.8–27.9) | .097 | – | .873 |
Were also included in the univariate analysis, but were not significant: age at diagnosis of SLE, age at diagnosis of cancer, smoking, alcohol, oral contraceptives, history of benign tumor, hypocomplementemia, Anti-DNA, Antiphospholipid antibodies, severe lupus and inmunosuppressive drugs (ever).
In the SLE cohort, 14 (8%) patients died, giving a mortality rate of 101 per 1000 patient-years (95% CI, 56–171). All the deaths were in women. The three main causes of death were cardiovascular events (36%), infection (29%) and disease activity (21%). No patient died due to a malignant tumour.
DiscussionThis study suggests that the incidence rate of cancer in our SLE patients is almost four times greater than expected for our hospital area of western Malaga. This finding was seen after observation of our cohort of SLE patients for 1370.5 patient-years, which is no different clinically or analytically from the large SLE series.1,14–19 Although the crude rate of cancer in the SLE patients was higher than that of the controls, the significance was lost after standardizing the rates. This is very likely due to the small sample size as compared with large multicentre studies. Another methodological factor that could influence in higher cancer rate of the SLE patients was a greater chance of detecting cancer in a prospective hospital cohort compared with controls that came freely. The same reason could be behind the observation that none of our lupus patient died from cancer.
The relation between cancer and immunologically mediated inflammatory diseases, including SLE, has been the subject of great debate for some time, and it has, consequently, been examined by many authors in several different countries. In 2005, Bernatsky et al. published the largest multicentre study, comprising 9547 patients from 23 centres in six different areas (Canada, United States, United Kingdom, Sweden, Korea and Iceland) and representing 76,948 patient-years, with a mean follow-up of 8 years.1 Like us, these authors also found an increased risk of cancer among SLE patients, though the risk was lower than in our study as they estimated a SIR of 1.15 (95% CI, 1.05–1.27). Prior to this study, many others had been done, with conflicting results. Some studies found a higher risk of cancer in patients with SLE,1,2,18 whereas several others failed to find this association,20,21 showing that this topic remains unresolved. This great variation in the results may have several explanations, including, importantly, the different distribution of the risk of cancer among the various countries (and even within the same country). Accordingly, it is even more important for each country to undertake its own studies on cancer incidence.
Another factor possibly influencing the variation in the results in cancer incidence concerns the study method employed. Some studies have involved a clinical group with no external control group, whereas others did use an external control group, either from a statistics centre,20,21 or a national/provincial cancer registry,17,18 and even a hospital registry,2 like that used by us.
The mean time between diagnosis of SLE and diagnosis of cancer in our cohort was 5 years. Bernatsky et al.,1 in a cohort of 9547 SLE patients, found that most of the cancers occurred after the first year of diagnosis of SLE. Nevertheless, considering that tumours have a very variable, prolonged subclinical period of development, the presence of SLE can hardly be held responsible solely for this greater incidence. This is so even though there is always a delay between the onset of lupus symptoms and its diagnosis, particularly in patients whose cancer is diagnosed during the initial years of SLE, which has led several authors to suggest that SLE may be considered a paraneoplastic syndrome in these patients.22 The genetic and immunological changes giving rise to SLE may also play a fundamental role in the development of malignant tumours many years before the disease is clinically expressed. To this extent, changes in immune vigilance and loss of tolerance may share certain mechanisms. In fact, cancer incidence was associated with chronic inflammation as determined by ESR in our patients. This association has been also observed in patients with rheumatoid arthritis.23 By contrast, the temporal association between cancer and thrombosis observed in our study could be explained by a dual mechanism, i.e., by the relationship between thrombosis and antiphospholipid antibodies in SLE and by thrombophilic state induced by the cancer as “second hit” that triggers thrombosis.24 Although we have not found an association between cancer and immunosuppressants, such as azathioprine or cyclophosphamide, it is worth mentioning that this association has been observed by others.25
The spectrum of malignant tumours in SLE patients is very variable. Almost all types of tumours have been reported,26 though the disease has most commonly been associated with haematological malignancies, mainly non-Hodgkin's lymphoma.1,14,19 The types of neoplasia that we observed were those expected for age and sex in general population. In our cohort we only found one pretumour haematological disorder, a myelodysplastic syndrome, which we did not include among the malignant tumours as it did not progress to acute leukaemia. Thus, we found no relation between SLE and haematological tumours, not even non-Hodgkin's lymphoma, as in other studies.18,21 Three main reasons may explain this lack of association. As Zintzaras et al. point out in their meta-analysis,27 the SIR has become lower over the years (in 1992 it was 44.40, in 1995 it was 27.10, and in 2001 it was 7.42), which would explain why our series, despite being an open observational study from 1992, found no lymphomas. Secondly, non-Hodgkin's lymphoma is usually more common in men28 and only 10% of our cohort was male. The third reason for the lack of association between SLE and haematological tumours relates to the association between lymphoma and inflammation. The improved control in SLE activity attained over recent years has contributed to lymphoma becoming an increasingly rarer complication, and sustained activity is related with the appearance of neoplasms and greater associated mortality.23
We want to emphasize that an important limitation of our study concerns the restricted number of patients, though not the observation period, which covered 17 years and resulted in a total observation of 1370.5 patient-years. Nevertheless, to reduce instability in the calculation of the stratum-specific rates associated with the observation of such a low number of events, we standardized the SIR by an indirect method, which assigned weights according to the stratum size of the SLE cohort, making them less susceptible to random variation. This aspect is important, because we saw that the SLE patients belonging to the younger age strata were those who had higher incidence rates of cancer, a finding not seen in other studies.21,29,30
In conclusion, the crude incidence rate of cancer in our cohort of SLE patients was almost four times greater than that seen in the equivalent population without SLE. Our results support the hypothesis that patients with SLE have a greater risk of cancer. A chronic increase in the ESR and the presence of thrombosis were associated with a greater risk of cancer in our SLE patients.
Ethical ResponsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Conflict of InterestThe authors declare no conflict of interest.