Periodontitis and rheumatoid arthritis (RA) have been associated in a bidirectional way. The objective of this study was to determine the association between clinical parameters of periodontitis and RA.
Materials and methodsSeventy-five (75) participants distributed in 3 groups (21 patients with periodontitis without RA, 33 patients with periodontitis with RA and 21 patients with reduced periodontium with RA) were included in this cross-sectional study. A full periodontal and medical examination was performed in each patient. Additionally, subgingival plaque samples for the detection of Porphyromonas gingivalis (P. gingivalis) and blood samples for biochemical markers of RA were also taken. Logistic regression analysis adjusted for confounding variables, Spearman's rank correlation coefficient and a linear multivariate regression were used to analyze the data.
ResultsPatients with RA presented less severity of periodontal parameters. The highest levels of anti-citrullinated protein antibodies were detected in non-periodontitis patients with RA. Covariates such as age, P. gingivalis, diabetes, smoking, osteoporosis and use of medication were not associated with RA. All periodontal variables and P. gingivalis expressed a negative correlation with biochemical markers of RA (P<0.05).
ConclusionsPeriodontitis was not associated with RA. Furthermore, there was no correlation between periodontal clinical parameters and biochemical markers of RA.
La periodontitis y la artritis reumatoide (AR) se han asociado de forma bidireccional. El objetivo de este estudio fue determinar la asociación entre parámetros clínicos de periodontitis y AR.
Materiales y métodosSetenta y cinco (75) participantes distribuidos en 3 grupos (21 pacientes con periodontitis sin AR, 33 pacientes con periodontitis con AR y 21 pacientes con periodonto reducido con AR) fueron incluidos en este estudio transversal. En cada paciente se realizó un examen médico y periodontal completo. Además, también se tomaron muestras de placa subgingival para la detección de Porphyromonas gingivalis(P. gingivalis) y muestras de sangre para marcadores bioquímicos de AR. Para analizar los datos se utilizó el análisis de regresión logística ajustado por variables de confusión, el coeficiente de correlación de rangos de Spearman y una regresión lineal multivariada.
ResultadosLos pacientes con AR presentaron menor severidad de los parámetros periodontales. Los niveles más altos de anticuerpos antiproteína citrulinada se detectaron en pacientes con AR sin periodontitis. Las covariables como la edad, P. gingivalis, diabetes, tabaquismo, osteoporosis y uso de medicamentos no se asociaron con la AR. Todas las variables periodontales y P. gingivalis expresaron una correlación negativa con los marcadores bioquímicos de AR (p<0,05).
ConclusionesLa periodontitis no se asoció con la AR. Además, no hubo correlación entre los parámetros clínicos periodontales y los marcadores bioquímicos de la AR.
Periodontitis is a chronic inflammatory process that starts as gingivitis and slowly progresses over time. It is initiated by a dysbiotic biofilm, but it can be modified by multiple factors such as diabetes and cigarette smoking. Due to its chronic nature, inflammation develops unnoticed leading to the loss of periodontal support and eventually, tooth loss. In addition, the effects of periodontitis not only affect the tooth support but accumulate with time. This causes systemic inflammatory changes that have associated periodontitis with systemic conditions such as pregnancy complications, cardiovascular disease, and rheumatoid arthritis. 1
Rheumatoid arthritis (RA) is also a chronic inflammatory disease of the joints with an autoimmune etiology. Pain and swelling of the joints ultimately lead to significant disability and reduced quality of life. RA is known to have a genetic basis, but it is also affected by environmental factors such as hormonal changes, diet, cigarette smoking and other medical conditions that include diabetes.2,3
Periodontitis and RA have similar immunologic features that result in the destruction of periodontal tissues and joints. Some authors have proposed a “two-hit” model where periodontitis represents the first hit and other unknown factors, the second hit that in the long run could influence the development of RA in a susceptible individual. 4 These features include high levels of inflammatory cytokines (IL-1, IL-6, IL-17, TNF) and systemic inflammatory biomarkers such as C-reactive protein (CRP).5 Although they have different etiologic origins, some studies suggest that periodontitis is associated with RA through the inflammatory axis and Porphyromonas gingivalis.6 The specific production of a peptidylarginine deiminase enzyme by P. gingivalis (PPAD), led to the proposal that the citrullination of proteins by PPAD at inflamed periodontal tissues could result in the production of anti-citrullinated protein antibodies (ACPAs) which is a key feature of RA. These pathological mechanisms are thought to be the connecting link between periodontitis and AR.
The systemic changes caused by periodontitis could have important detrimental effects on the health of individuals. Results from the First National Health and Nutrition Examination Survey (NHANES I) and its epidemiological follow-up study (NHEFS) showed that participants with periodontal disease had higher odds of prevalent/incident RA.7 Nevertheless, the odds ratios (ORs) were not statistically significant. In contrast, other studies have suggested that periodontitis is a significant risk factor for RA resulting in controversial results.8,9 Therefore, since the association between periodontitis and RA has not been fully clarified, this study aimed to determine the association between clinical parameters of periodontitis and rheumatoid arthritis.
Materials and methodsStudy design, setting and ethical statementThis cross-sectional study involved patients that consulted at the dental clinics of the Facultad de Odontología (Universidad de Antioquia, Colombia) and a clinic for rheumatoid arthritis (Artmédica, Colombia). The study protocol was approved by the institutional review board of the Facultad de Odontología (Universidad de Antioquia, Colombia; 05-2016) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. Informed consent was obtained from all individual participants included in the study. In addition, the guidelines of The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement were followed.
Population and sampleIndividuals with and without RA were invited to participate between March 2019 and March 2020. The sample consisted of 75 participants distributed in 3 groups: 21 patients with periodontitis without RA, 33 patients with periodontitis and RA, and 21 patients with reduced periodontium and RA. The latter group included subjects with clinical gingival health/gingivitis on a reduced periodontium in stable periodontitis or non-periodontitis patients.
Selection criteriaPotential participants were included if they met the following criteria: ≥18 years old; confirmed diagnosis of RA according to the American College of Rheumatology with a disease activity score-28 (DAS28-CRP) ≥3.2 and no change in RA medication in the previous 3 months and at least 15 teeth excluding third molars.10 Participants without RA met the same inclusion criteria except for the parameters and confirmed diagnosis of RA. On the other hand, potential participants were excluded when they had: periodontal treatment or use of antibiotics in the previous 3 months, uncontrolled diabetes, HIV, liver disease, head and neck radiation therapy, pregnancy and use of cyclosporine. Smoking, hypertension, and hyperlipidemia medication were not considered reasons for exclusion and were recorded accordingly for analysis.
Clinical examinationA full periodontal examination was carried out by a trained single clinician using a periodontal probe (UNC-15) and recording the gingival margin, probing depth (PD), periodontal attachment level (CAL) and bleeding on probing (BoP) at six sites per tooth excluding third molars. Periodontitis was defined as 2 or more non-adjacent interdental sites with loss of CAL, PD≥4mm, and BoP. The stage of periodontitis was determined according to the current classification for periodontal diseases.11 Clinical gingival health/gingivitis on a reduced periodontium in stable periodontitis or non-periodontitis patients were characterized by loss of CAL, PD≤3mm, and BoP.12 In addition, the Periodontal Inflammatory Burden Index (PIBI) was calculated as previously reported.13 The clinical investigator who recorded periodontal parameters was calibrated for repeated measurements before patient inclusion (Kappa value was ≥0.80 for CAL and PD).
Subgingival plaque samples from the 5 deepest sites were taken using sterile paper points inserted to the bottom of the sulcus/pocket for 30s and pooled in a vial containing VMGA III transport medium (Viability Medium Götenborg Anaerobical). All samples were processed within 24h and incubated in anaerobic culture chambers using Brucella blood agar for the detection of P. gingivalis.
The levels of anti-citrullinated protein antibodies (ACPAs; U/mL), rheumatoid factor (RF; U/mL) and high-sensitivity C-reactive protein (hs-CRP; mg/L) were measured in blood samples from all participants in a reference laboratory by standard methods.
A trained rheumatology doctor examined patients with RA during the study and confirmed the diagnosis. Medical information from was obtained from their medical records which included: duration of RA (DAS28-CRP) and current medication for RA (nonsteroidal anti-inflammatory drugs (NSAIDs), non-biologic and biologic disease-modifying antirheumatic drugs (DMARDs) and corticosteroids). Non-biologic DMARDs included hydroxychloroquine, methotrexate, sulfasalazine and leflunomide. Biologic DMARDs included Adalimumab, Etanecerpt, Abatacept, Golimumab, Infliximab, Rituximab and Tocilizumab.
Microbial detectionMicrobial detection was performed using anaerobic culture methods of subgingival plaque samples as described by Botero et al. (2007).14 The detection of P. gingivalis, was based on colony morphology and by standard biochemical tests. Microbial counts greater than 0 colony forming units per mL (CFU/mL) were considered a positive detection.
Data analysisAn initial exploratory and descriptive analysis was carried out to determine the distribution of variables and proper tests (Kolmogorov–Smirnov) were conducted to assess for normality. Continuous variables are presented with the corresponding central tendency and dispersion measures and parametric or non-parametric tests were used to determine differences between groups where indicated. Categorical variables are presented as the frequency and differences between groups were tested with the Chi2.
Logistic regression analysis was carried to test for the association between covariates and RA. The crude odds ratio (OR 95% confidence interval) and adjusted ORs for covariates are presented in the multivariate analysis included sex, age, osteoporosis, NSAIDs, corticosteroids and anti-hypertensive medication according to the Hosmer–Lemeshow criterion (P≤0.25). Furthermore, to determine the influence of periodontal variables (independent variables) on biochemical markers of RA (dependent variables), the Spearman's rank correlation coefficient and a linear multivariate regression analysis was carried out to evaluate the simultaneous and reciprocal effect of the explanatory variables on each one of the biochemical markers of RA. The compliance with the assumptions of linearity, non-collinearity and normality, constant variance and correlation of residuals was determined. The calculated sample size was 76 patients with a 95% confidence interval, a 5% error, an expected prevalence of 20%, for a study power of 80%. All data was analyzed in SPSS (IBM SPSS Statistics for Windows, Version 25) and statistical significance was assumed when P≤0.05.
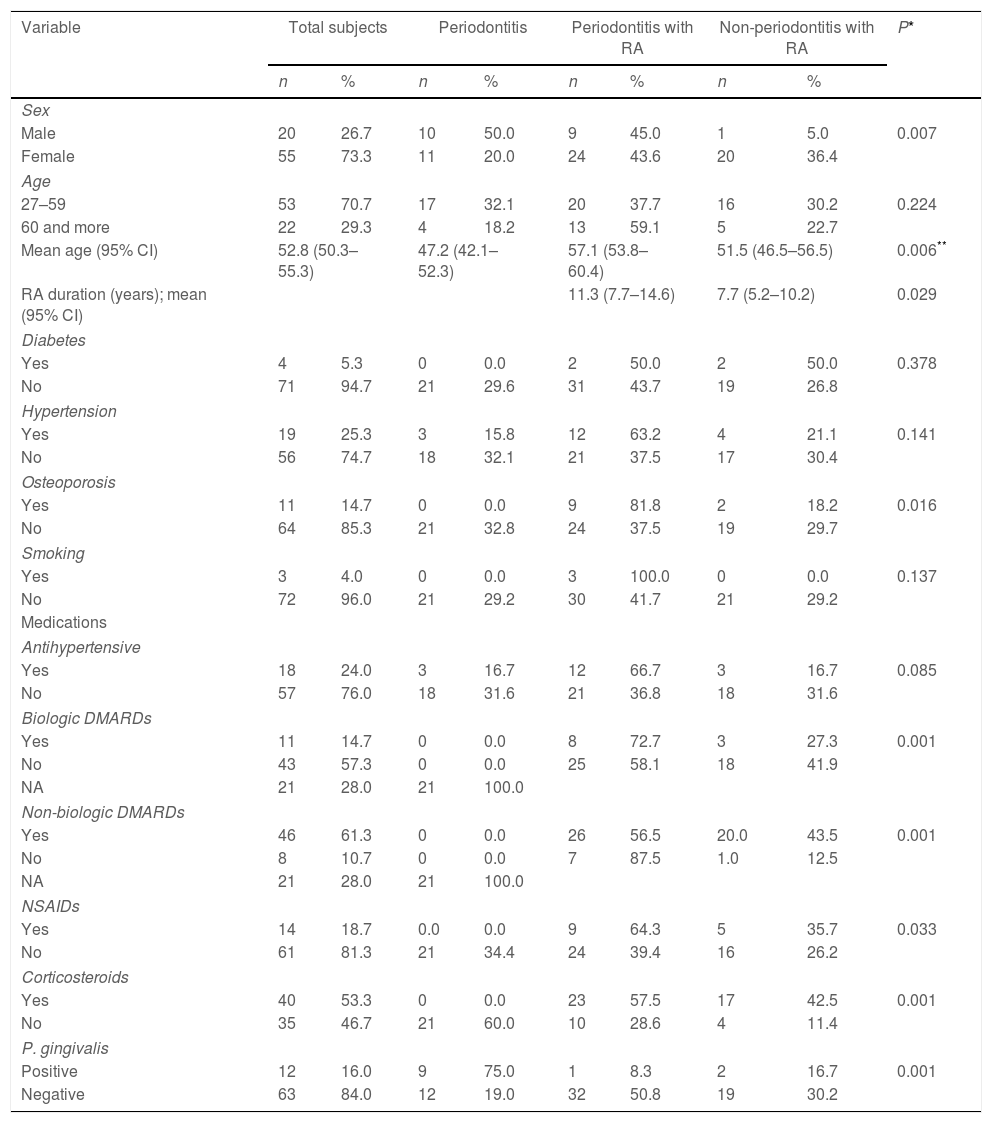
ResultsTable 1 lists the demographic and clinical characteristics of the participants of the study. There were more female participants, and this was specially observed in the RA groups (P<0.05). The mean age of participants was 52.8 years old (95% CI: 50.3–55.3) but periodontitis patients without RA were younger than RA patients (P<0.05). The duration of RA was between 5 and 14 years although patients with periodontitis had higher duration (11.3; 95% CI: 7.7–14.6) than non-periodontitis patients (P<0.05). No differences regarding the frequency of diabetes, hypertension and smoking were observed. In contrast, osteoporosis was more frequent in RA patients (P<0.05). The use of hypertensive medication was similar in all groups. Medications for RA which include DMARDs, NSAIDs and corticosteroids are reported.
Demographic and clinical characteristics of the study sample.
| Variable | Total subjects | Periodontitis | Periodontitis with RA | Non-periodontitis with RA | P* | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Sex | |||||||||
| Male | 20 | 26.7 | 10 | 50.0 | 9 | 45.0 | 1 | 5.0 | 0.007 |
| Female | 55 | 73.3 | 11 | 20.0 | 24 | 43.6 | 20 | 36.4 | |
| Age | |||||||||
| 27–59 | 53 | 70.7 | 17 | 32.1 | 20 | 37.7 | 16 | 30.2 | 0.224 |
| 60 and more | 22 | 29.3 | 4 | 18.2 | 13 | 59.1 | 5 | 22.7 | |
| Mean age (95% CI) | 52.8 (50.3–55.3) | 47.2 (42.1–52.3) | 57.1 (53.8–60.4) | 51.5 (46.5–56.5) | 0.006** | ||||
| RA duration (years); mean (95% CI) | 11.3 (7.7–14.6) | 7.7 (5.2–10.2) | 0.029 | ||||||
| Diabetes | |||||||||
| Yes | 4 | 5.3 | 0 | 0.0 | 2 | 50.0 | 2 | 50.0 | 0.378 |
| No | 71 | 94.7 | 21 | 29.6 | 31 | 43.7 | 19 | 26.8 | |
| Hypertension | |||||||||
| Yes | 19 | 25.3 | 3 | 15.8 | 12 | 63.2 | 4 | 21.1 | 0.141 |
| No | 56 | 74.7 | 18 | 32.1 | 21 | 37.5 | 17 | 30.4 | |
| Osteoporosis | |||||||||
| Yes | 11 | 14.7 | 0 | 0.0 | 9 | 81.8 | 2 | 18.2 | 0.016 |
| No | 64 | 85.3 | 21 | 32.8 | 24 | 37.5 | 19 | 29.7 | |
| Smoking | |||||||||
| Yes | 3 | 4.0 | 0 | 0.0 | 3 | 100.0 | 0 | 0.0 | 0.137 |
| No | 72 | 96.0 | 21 | 29.2 | 30 | 41.7 | 21 | 29.2 | |
| Medications | |||||||||
| Antihypertensive | |||||||||
| Yes | 18 | 24.0 | 3 | 16.7 | 12 | 66.7 | 3 | 16.7 | 0.085 |
| No | 57 | 76.0 | 18 | 31.6 | 21 | 36.8 | 18 | 31.6 | |
| Biologic DMARDs | |||||||||
| Yes | 11 | 14.7 | 0 | 0.0 | 8 | 72.7 | 3 | 27.3 | 0.001 |
| No | 43 | 57.3 | 0 | 0.0 | 25 | 58.1 | 18 | 41.9 | |
| NA | 21 | 28.0 | 21 | 100.0 | |||||
| Non-biologic DMARDs | |||||||||
| Yes | 46 | 61.3 | 0 | 0.0 | 26 | 56.5 | 20.0 | 43.5 | 0.001 |
| No | 8 | 10.7 | 0 | 0.0 | 7 | 87.5 | 1.0 | 12.5 | |
| NA | 21 | 28.0 | 21 | 100.0 | |||||
| NSAIDs | |||||||||
| Yes | 14 | 18.7 | 0.0 | 0.0 | 9 | 64.3 | 5 | 35.7 | 0.033 |
| No | 61 | 81.3 | 21 | 34.4 | 24 | 39.4 | 16 | 26.2 | |
| Corticosteroids | |||||||||
| Yes | 40 | 53.3 | 0 | 0.0 | 23 | 57.5 | 17 | 42.5 | 0.001 |
| No | 35 | 46.7 | 21 | 60.0 | 10 | 28.6 | 4 | 11.4 | |
| P. gingivalis | |||||||||
| Positive | 12 | 16.0 | 9 | 75.0 | 1 | 8.3 | 2 | 16.7 | 0.001 |
| Negative | 63 | 84.0 | 12 | 19.0 | 32 | 50.8 | 19 | 30.2 | |
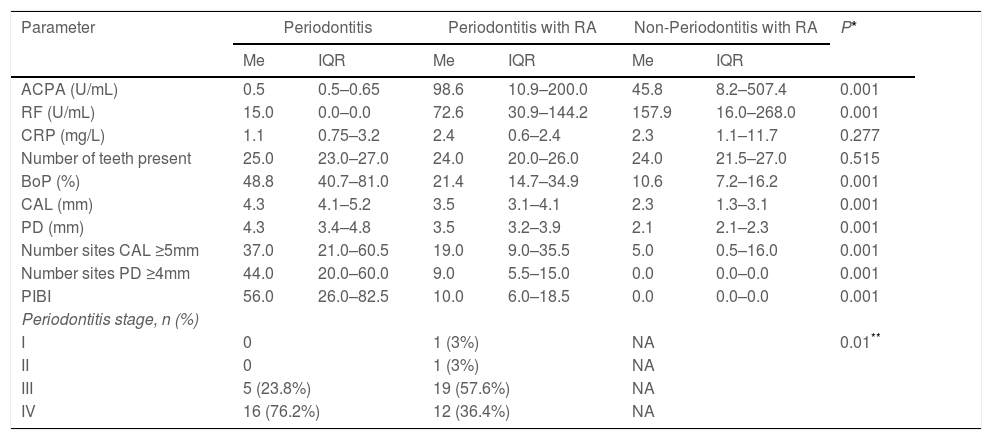
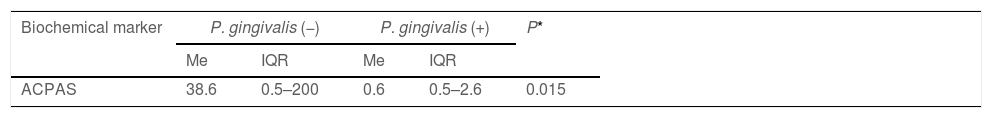
In general, participants were comparable regarding the number of teeth present (Table 2). Nonetheless, the periodontitis group presented mainly stage IV periodontitis with worse periodontal condition characterized by increased loss of CAL, presence of periodontal pockets, BoP, and periodontal inflammatory burden than patients with RA and this difference was statistically significant (P<0.05). Participants in the periodontitis with RA group presented less periodontal destruction while the non-periodontitis with RA group presented minimal inflammation on a reduced periodontium. The highest levels of ACPAs and RF were detected in non-periodontitis patients with RA followed by periodontitis patients with RA (P<0.05). The levels of ACPAs and RF were minimal in participants without RA (P<0.05). A similar trend was observed regarding CRP levels, but the difference was not statistically significant. The levels of ACPAs were higher in P. gingivalis negative patients as compared to P. gingivalis positive patients (P<0.05; Table 3).
Biochemical markers of RA and periodontal clinical parameters of the study groups.
| Parameter | Periodontitis | Periodontitis with RA | Non-Periodontitis with RA | P* | |||
|---|---|---|---|---|---|---|---|
| Me | IQR | Me | IQR | Me | IQR | ||
| ACPA (U/mL) | 0.5 | 0.5–0.65 | 98.6 | 10.9–200.0 | 45.8 | 8.2–507.4 | 0.001 |
| RF (U/mL) | 15.0 | 0.0–0.0 | 72.6 | 30.9–144.2 | 157.9 | 16.0–268.0 | 0.001 |
| CRP (mg/L) | 1.1 | 0.75–3.2 | 2.4 | 0.6–2.4 | 2.3 | 1.1–11.7 | 0.277 |
| Number of teeth present | 25.0 | 23.0–27.0 | 24.0 | 20.0–26.0 | 24.0 | 21.5–27.0 | 0.515 |
| BoP (%) | 48.8 | 40.7–81.0 | 21.4 | 14.7–34.9 | 10.6 | 7.2–16.2 | 0.001 |
| CAL (mm) | 4.3 | 4.1–5.2 | 3.5 | 3.1–4.1 | 2.3 | 1.3–3.1 | 0.001 |
| PD (mm) | 4.3 | 3.4–4.8 | 3.5 | 3.2–3.9 | 2.1 | 2.1–2.3 | 0.001 |
| Number sites CAL ≥5mm | 37.0 | 21.0–60.5 | 19.0 | 9.0–35.5 | 5.0 | 0.5–16.0 | 0.001 |
| Number sites PD ≥4mm | 44.0 | 20.0–60.0 | 9.0 | 5.5–15.0 | 0.0 | 0.0–0.0 | 0.001 |
| PIBI | 56.0 | 26.0–82.5 | 10.0 | 6.0–18.5 | 0.0 | 0.0–0.0 | 0.001 |
| Periodontitis stage, n (%) | |||||||
| I | 0 | 1 (3%) | NA | 0.01** | |||
| II | 0 | 1 (3%) | NA | ||||
| III | 5 (23.8%) | 19 (57.6%) | NA | ||||
| IV | 16 (76.2%) | 12 (36.4%) | NA | ||||
Chi2 test. U: international units, ACPA: anti-citrullinated protein antibody, RF: rheumatoid factor, CRP: C-reactive protein, BoP: bleeding on probing, CAL: clinical attachment level, PD: probing depth, PIBI: periodontal inflammation burden index, Me: median, IQR: interquartile range, NA: not applicable.
Levels of ACPAs according to the presence of P. gingivalis.
| Biochemical marker | P. gingivalis (−) | P. gingivalis (+) | P* | ||
|---|---|---|---|---|---|
| Me | IQR | Me | IQR | ||
| ACPAS | 38.6 | 0.5–200 | 0.6 | 0.5–2.6 | 0.015 |
The association between covariates and RA is presented in Table 4. Covariates such as age, P. gingivalis, diabetes, smoking, osteoporosis, and use of medication were not associated with RA. The model explained 90.3% in variance of the dependent variable (RA).
Logistic regression analyses for the association between covariates and RA.
| Covariate | Crude OR (95% CI) | Adjusted OR (95% CI)* |
|---|---|---|
| Sex | ||
| Male | 1.0 | |
| Female | 4.0 (1.3–11.9) | 1.9 (0.1–26.1) |
| Age | ||
| 27–59 | 1.0 | |
| 60 and more | 2.1 (0.6–7.2) | 2.2 (0.2–24.7) |
| P. gingivalis | ||
| Yes | 0.078 (0.1–0.3) | |
| No | 1.0 | |
| Smoking | ||
| Yes | 0.7 (0.6–0.8) | |
| No | 1.0 | |
| Diabetes | ||
| Yes | 0.7 (0.6–0.8) | |
| No | 1.0 | |
| Hypertension | ||
| Yes | 2.5 (0.6–9.7) | |
| No | 1.0 | |
| Anti-hypertensive | ||
| Yes | 2.3 (0.5–8.9) | 0.1 (0.0–1.4) |
| No | 1.0 | |
| Osteoporosis | ||
| Yes | 0.7 (0.6–0.8) | |
| No | 1.0 | |
| NSAIDs | ||
| Yes | 0.7 (0.6–0.8) | |
| No | 1.0 | |
| Corticosteroids | ||
| Yes | 0.4 (0.3–0.6) | |
| No | 1.0 | |
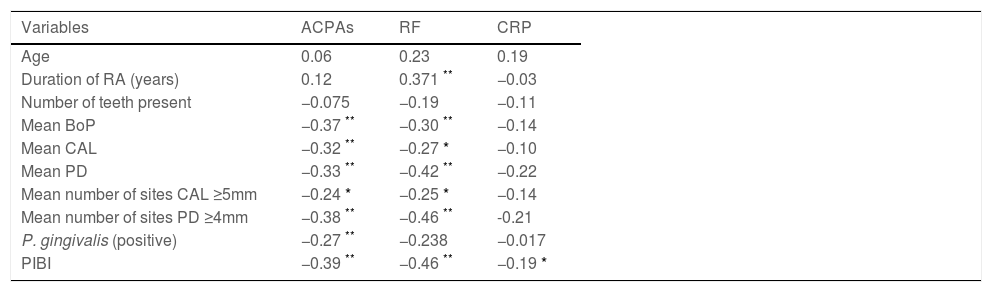
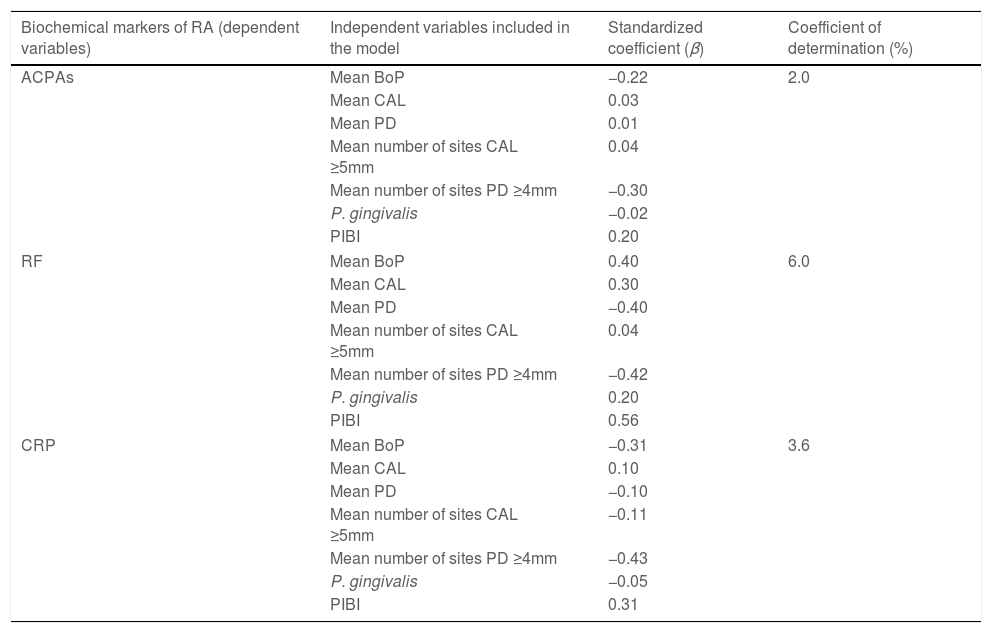
The correlation between relevant clinical variables and biochemical markers of RA is presented in Table 5. In general, all periodontal variables expressed a negative correlation with ACPAs, RF and CRP (P<0.05). Age presented a slight positive correlation with biochemical markers of AR but was not statistically significant. The duration of RA was positively correlated with ACPAs and specially with RF (P<0.05) but not with CRP. Furthermore, in the multivariate model (Table 6), periodontal clinical variables such as CAL, PD and PIBI presented correlation coefficients were less than 0.50, suggesting that these indicators could only partially explain the correlation between periodontitis and ACPAs and CRP. The detection of P. gingivalis was a negative predictor variable for ACPAs in the model. Conversely, P. gingivalis was a positive predictor variable for RF. The highest statistical weight observed was between periodontal variables and RF. The independent variables described explained between 2 and 6% of the scores obtained for RA biomarkers. Nonetheless, these correlations did not reach statistical significance.
Correlation between relevant variables and biochemical markers of RA.
| Variables | ACPAs | RF | CRP |
|---|---|---|---|
| Age | 0.06 | 0.23 | 0.19 |
| Duration of RA (years) | 0.12 | 0.371 ** | −0.03 |
| Number of teeth present | −0.075 | −0.19 | −0.11 |
| Mean BoP | −0.37 ** | −0.30 ** | −0.14 |
| Mean CAL | −0.32 ** | −0.27 * | −0.10 |
| Mean PD | −0.33 ** | −0.42 ** | −0.22 |
| Mean number of sites CAL ≥5mm | −0.24 * | −0.25 * | −0.14 |
| Mean number of sites PD ≥4mm | −0.38 ** | −0.46 ** | -0.21 |
| P. gingivalis (positive) | −0.27 ** | −0.238 | −0.017 |
| PIBI | −0.39 ** | −0.46 ** | −0.19 * |
ACPA: anti-citrullinated protein antibody, RF: rheumatoid factor, CRP: C-reactive protein, BoP: bleeding on probing, CAL: clinical attachment level, PD: probing depth, PIBI: periodontal inflammatory burden index. Spearman's rank correlation coefficient.
Multivariate linear regression model for the correlation of periodontal variables and biochemical markers of RA.
| Biochemical markers of RA (dependent variables) | Independent variables included in the model | Standardized coefficient (β) | Coefficient of determination (%) |
|---|---|---|---|
| ACPAs | Mean BoP | −0.22 | 2.0 |
| Mean CAL | 0.03 | ||
| Mean PD | 0.01 | ||
| Mean number of sites CAL ≥5mm | 0.04 | ||
| Mean number of sites PD ≥4mm | −0.30 | ||
| P. gingivalis | −0.02 | ||
| PIBI | 0.20 | ||
| RF | Mean BoP | 0.40 | 6.0 |
| Mean CAL | 0.30 | ||
| Mean PD | −0.40 | ||
| Mean number of sites CAL ≥5mm | 0.04 | ||
| Mean number of sites PD ≥4mm | −0.42 | ||
| P. gingivalis | 0.20 | ||
| PIBI | 0.56 | ||
| CRP | Mean BoP | −0.31 | 3.6 |
| Mean CAL | 0.10 | ||
| Mean PD | −0.10 | ||
| Mean number of sites CAL ≥5mm | −0.11 | ||
| Mean number of sites PD ≥4mm | −0.43 | ||
| P. gingivalis | −0.05 | ||
| PIBI | 0.31 | ||
ACPA: anti-citrullinated protein antibody; RF: rheumatoid factor; CRP: C-reactive protein; PIBI: periodontal inflammatory burden index.
The results of our study produced contrasting results on the association between periodontitis and RA as opposed to previous studies. However, several aspects related to the study of periodontitis and systemic relationships warrant discussion. It is our contention not to refute previous evidence, but to show that perhaps, the association between periodontitis and RA is not clear.
The periodontal condition of periodontitis patients without RA was significantly worse than patients with RA, even with periodontitis. This means higher loss of CAL, deep periodontal pockets, and BoP. In this study, we defined a case of periodontitis as the presence of at least 2 interproximal non-adjacent sites with concurrent loss of CAL, PD≥4mm and BoP. This definition was based on the latest classification of periodontal diseases to differentiate between periodontitis and a reduced periodontium. Previous studies that have reported an association between periodontitis and RA, defined periodontitis on the sole basis of loss of CAL. But loss of CAL represents previous multifactorial exposures and is not exclusive of periodontitis. In fact, the frequency of sites with loss of CAL increases with age and it is now accepted that a healthy/stable periodontium can exist in the presence of loss of CAL and a probing of ≤4mm without BoP.11 For argumentation purposes, we even analyzed the data considering all patients with RA as having periodontitis, but the periodontal parameters were still lower than patients without RA. Although loss of CAL is a sequelae of periodontitis, it is the periodontal pocket (≥4mm with BoP) the sign of current periodontitis. This marks a clear difference between our study and others and the resulting groups for analysis.
We could not find evidence that patients with RA have a more severe periodontitis or loss of CAL than patients without RA as previous reports have suggested.15,16 However, it is worth noting that such studies reported a <0.5mm difference in CAL and PD in subjects with RA as compared to subjects without RA, which is not considered clinically significant. As it occurs in most of the studies, patients with RA are recruited from specialized rheumatology clinics. The same occurs for periodontitis patients from dental clinics. Inclusion parameters were defined to select patients with and without RA and afterwards the periodontal condition was determined. Even though all groups were comparable regarding age and number of teeth, periodontal parameters were more severe in subjects without RA. This may be due to the fact that our patients without RA resulted from the dental clinics of a public university where people with low income are seeking treatment for their dental condition and have limited access to health services. In comparison, RA patients were monitored periodically at their institution and had better access to medical and dental treatments. Hence, it is possible that appropriate access to health services influenced the periodontal condition.
The chronic use of special RA medication could have an impact on periodontal parameters. The results from our study, showed that patients with RA presented less CAL and this difference may be due to the chronic use (>5 years) of DMARDs which was exclusive of RA patients. Studies in animals have shown that the administration of chloroquine reduces periodontal inflammation and bone loss in experimental periodontitis.17 Furthermore, studies in humans revealed that the use of DMARDs and corticosteroids are associated with improvement in CAL in patients with RA and ankylosing spondylitis18,19 DMARDs have potent anti-inflammatory effects and have been recently considered as prospects co-adjuvants in the treatment of periodontitis.20 The study by Heredia-P et al. (2019) showed that DMARD medication in early RA patients was associated with less progression in the loss of CAL after 1-year follow up period suggesting a possible effect on periodontal parameters.21 Similar results were obtained by Rovas et al. (2021) and Punceviciene et al. (2021).16,22 However, the mechanisms of this phenomenon are unknown and could not be analyzed in our study.
We could not demonstrate that periodontitis was associated with RA as previously suggested. A study in 75 patients with RA, 60% presented periodontitis but there was no association between probing depths and disease activity of RA.23 The study by Chen et al. (2013) in a large population-based study reported that the association was still weak after adjusting for confounding variables (OR 1.16; 95% CI 1.12–1.20).24 Another large population-based study revealed no evidence of an increased prevalence of periodontitis in patients with established RA compared to healthy controls.25 But demonstrating the bidirectional relationship between periodontitis and RA is still controversial as the results of the combined evidence does not have a strong correlation between periodontitis and biochemical markers of RA such as ACPAs.23 Wen et al. (2019) found that not all studies report a significant association between periodontal parameters and RA and therefore the results are inconclusive.26 A recent systematic review and meta-analysis showed that there is no substantial effect (<0.5mm) of RA on periodontal probing depth and CAL after combining the available studies.27 In addition, another systematic review and meta-analysis that evaluated whether periodontitis represents a risk for RA, reported higher prevalence of RA among patients with periodontitis as compared to controls (OR 1.97; 95% CI 1.68–2.31).28 Nonetheless, the association is moderate and stems from few high heterogeneity studies (I2=96%). Strong associations are generally accepted with ORs greater than 3 and no study has reliably proven the association between periodontitis and RA. In addition, the detection of P. gingivalis does not support its role in RA in this study. P. gingivalis is an important periodontal pathogen that produces a peptidylarginine deiminase enzyme (PPAD) that induce ACPAs formation and therefore could aggravate RA. Another periodontal pathogen such as Aggregatibacter actinomycetemcomitans has also been recently implicated with the development of RA through the hyperactivation of host peptidylarginine deiminase enzymes and therefore suggesting a more complex interaction between periodontal pathogens and the host.29,30 However, the role of these periodontal pathogens still warrants further studies. In general, the collective evidence gathered to this day is controversial due to consistency, strength, and specificity, leaving the association between periodontitis and RA inconclusive for the moment.
ConclusionsPeriodontitis was not associated with RA. Furthermore, there was no correlation between periodontal clinical parameters and biochemical markers of RA.
Author contributionsAPL, EBG and JEB contributed to the concept and design of the study. EBG, RAP and JEB contributed to the methodology of the study. AP and JEB contributed to the analysis of data. All authors contributed equally to the drafting of the manuscript.
EthicsThe study was reviewed and approved by the institutional review board (05-2016).
FundingThis study was funded by a grant [CODI 2015-6263] from the Universidad de Antioquia.
Conflict of interestsDr. Botero received a grant from Universidad de Antioquia. The rest of the authors have nothing to declare.