Familial Mediterranean fever (FMF) and systemic lupus erythematosus (SLE) are autosomal recessive auto-inflammatory diseases, triggered by FMF-associated gene mutations and auto-antigens. The literature on the co-occurrence of these two disorders is limited to case reports and their correlation is considered rare. We investigated the proportion of FMF among SLE patients when compared with a healthy adult cohort in South Asia.
MethodsFor this observational study, data from our institutional database were collected for the patients diagnosed with SLE. The control group was randomly selected from the database and were age- matched for SLE. The overall proportion of FMF among patients with and without SLE was considered. Student's t-test, Chi-square, and ANOVA were used for univariate analysis.
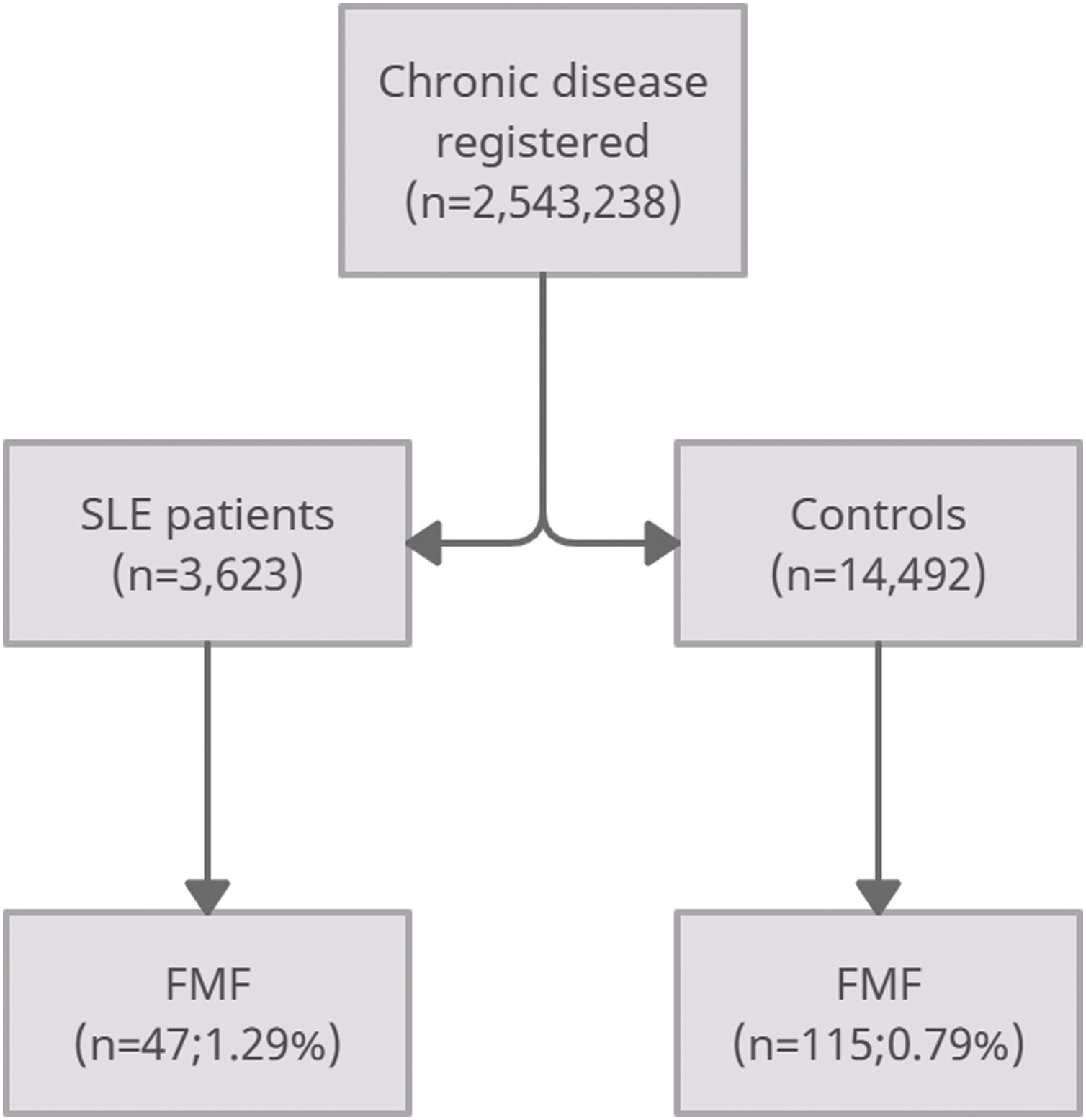
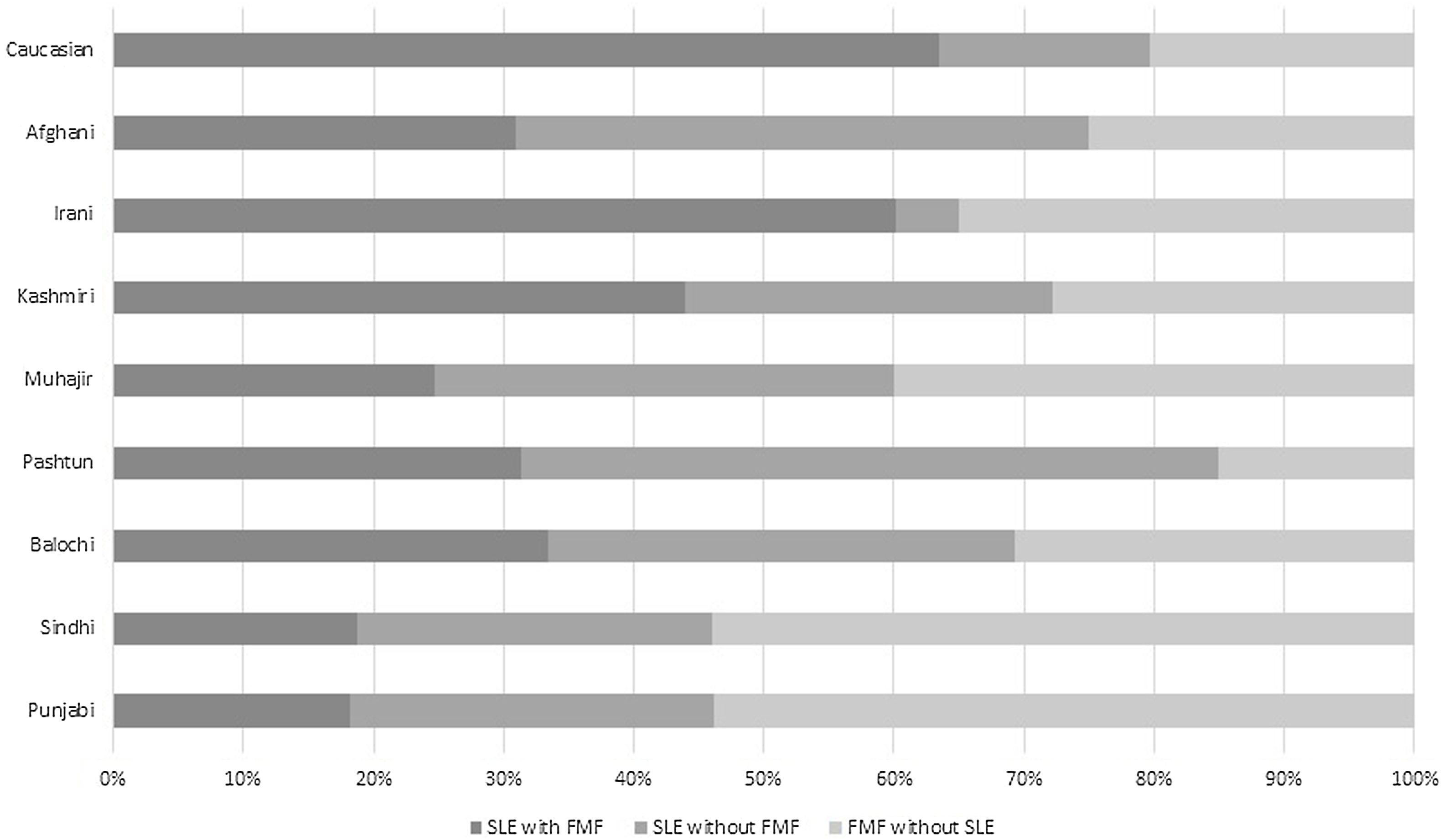
ResultsThe study population included 3623 SLE patients and 14,492 controls. In the SLE group, there was a significantly higher proportion of FMF patients compared with the non-SLE group (1.29% vs. 0.79% respectively; p=0.015). SLE was prevalent in Pashtun's (50%) in the middle socioeconomic group while FMF was dominant in Punjabi's and Sindhi's (53%) in the low socioeconomic class.
ConclusionThis investigation demonstrates that FMF is more prevalent in a South-Asian population cohort of SLE patients.
La fiebre mediterránea familiar (FMF) y el lupus eritematoso sistémico (LES) son enfermedades autoinflamatorias autosómicas recesivas, desencadenadas por mutaciones genéticas asociadas a la FMF y autoantígenos. La literatura sobre la coexistencia de estos dos trastornos se limita a informes de casos y su correlación se considera rara. Investigamos la proporción de FMF entre pacientes con LES en comparación con una cohorte de adultos sanos en el sudeste asiático.
MétodosPara este estudio observacional se recolectaron datos de nuestra base de datos institucional para los pacientes diagnosticados con LES. El grupo control se seleccionó al azar de la base de datos y se emparejaron por edad y sexo para LES. Se consideró la proporción global de FMF entre pacientes con y sin LES. Se utilizaron la prueba t de Student, χ2 y ANOVA para el análisis univariado.
ResultadosLa población de estudio incluyó a 3.623 pacientes con LES y 14.492 controles. En el grupo de LES, hubo una proporción significativamente mayor de pacientes con FMF en comparación con el grupo sin LES (1,29 vs. 0,79%, respectivamente; p=0,015). LES prevaleció en la región pastún (50%) en el grupo socioeconómico medio, mientras que FMF fue dominante en Punyab y Sind (53%) en la clase socioeconómica baja.
ConclusiónEsta investigación demuestra que la FMF es más frecuente en una cohorte de pacientes con LES del sudeste asiático.
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disease characterized as a chronic condition with autoantibody production against several organ systems of the body.1 Polyserositis, including pericarditis, pleuritis, and peritonitis is a common feature of SLE.2 SLE is characterized by immune dysregulation and the production of aberrant auto-antibodies.3 Although the exact pathophysiology in SLE still needs to be clarified, dysfunction of innate and adaptive immune system activation is well acknowledged.4 The heterogeneity of organ system involvement in SLE has been recognized for two decades by clinicians and scientists. With contemporary detection techniques and updated diagnostic criteria, disease burden and mortality associated with SLE have declined dramatically. For instance, a 10-year survival has increased from 63.2% in the 1950s to 95% in modern-day patients.5 SLE is associated with connective tissue diseases of chronic inflammation and auto-antigenicity potential and case reports describe its association with Familial Mediterranean fever (FMF).6
FMF is a multi-system disease inherited as an autosomal recessive trait, characterized as a fever syndrome with multiple organ involvement. It is distinguished by recurrent attacks of spontaneous peritonitis, pleuritis, and arthritis.7 However, in contrast to a sporadic geographic distribution of SLE, FMF is specifically seen in ethnic groups of Mediterranean origin.8 It is a genetic disorder with the MEFV gene as the central cause for the pathogenesis of FMF, which encodes the pyrin protein involved in inflammation.9 Because of the symptom overlap between SLE and FMF, the literature on the coexistence of both diseases is inconsistent and consists mostly of case reports.10–12 Therefore, we investigated the coexistence of these two chronic inflammatory diseases in this study and sought to determine if FMF is more common in patients with SLE compared with the general population. This is the first study to be conducted on a South-Asian population.
MethodsThis observational study was conducted at Foundation University using the institutional rheumatology patient database. Our institute has detailed, cloud-based data with continuous input from physicians and administration about patient status. Ethical review committee waived off patient consent for anonymized data presented in the study (Study ID # FFH/21/DCA/007). In this database, the diagnosis of chronic diseases is entered after confirmation of disease through various tests ordered by physicians at our institute. Disease status is divided into working diagnosis, probable diagnosis, and final diagnosis. Once the diagnosis is established, the primary physician can mark the final diagnosis in the system.
In this investigation, patients were defined as having SLE and FMF when their medical record contained the final diagnosis of either condition by the primary physician. Since the aim of this study was to reflect the co-occurrence in real-world population, we based our research on specialist diagnosis according to the 2019 European League Against Rheumatism (EULAR)/American College of Rheumatology (ACR) classification criteria for SLE and diagnostic criteria of FMF by Livneh et al.13,14 Patients diagnosed as definite SLE were included while patients falling under probable, possible, and undifferentiated connective tissue disease (CTD) were excluded. The control group was randomly selected from the database and were age-matched for SLE (1:4). Four groups were divided into controls with FMF, controls without FMF, SLE with FMF, and SLE without FMF. Regional variation was also noted during data collection to assess the prevalence of SLE, FMF or both in our patient pool.
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software version 26 (IBM Corp, Armonk, NY, USA.). Continuous variables were presented as mean±standard deviation (SD) and categorical variables as frequency and percentages. Continuous variables were analyzed using Student's t-test and categorical variables with Chi-square test. A p-value of less than 0.05 was considered significant.
ResultsUsing the institutional database, we retrieved full diagnosis of SLE in 3623 patients and 14,492 age-matched controls. Patients with SLE had female predominance (84%), with a mean age of 55±12 years. The SLE group had a significantly higher proportion of FMF patients compared with controls without SLE (1.29% vs. 0.79% respectively; p-value=0.015) (Fig. 1).
As for comorbid conditions, hypertension (HTN) was more prevalent in controls with FMF (36.5%) followed by SLE with FMF (36.1%), while chronic kidney disease (CKD) (7.6%), cerebrovascular accidents (CVAs) (9.6%), peripheral arterial disease (PAD) (10.5%), and venous thromboembolism (VTE) (8.5%) were dominant in SLE without FMF group (Tables 1 and 2).
Demographics and clinical characteristics of SLE and controls without FMF.
| Variables | Controls without FMF (n=14,377) | SLE without FMF (n=3576) | p-value |
|---|---|---|---|
| Age; years (mean±SD) | 58±11 | 55±12 | 0.045 |
| Females; n (%) | 4834 (33.6%) | 3004 (84%) | <0.001 |
| Comorbids | |||
| DM; n (%) | 4354 (30.2%) | 1042 (29.1%) | 0.321 |
| HTN; n (%) | 4109 (28.5%) | 1207 (33.7%) | 0.131 |
| CKD; n (%) | 565 (3.9%) | 274 (7.6%) | 0.036 |
| CVA; n (%) | 329 (2.2%) | 346 (9.6%) | <0.001 |
| CAD; n (%) | 3654 (25.4%) | 995 (27.8%) | 0.119 |
| PAD; n (%) | 371 (2.5%) | 376 (10.5%) | 0.002 |
| VTE; n (%) | 175 (1.2%) | 304 (8.5%) | <0.001 |
| Thyroid disease; n (%) | 578 (4%) | 145 (4%) | 1 |
| Cancer; n (%) | 155 (1%) | 34 (0.9%) | 0.953 |
| Socioeconomic status | |||
| Low; n (%) | 7351 (51.1%) | 1126 (31.4%) | 0.003 |
| Medium; n (%) | 4621 (32.1%) | 1476 (41.2%) | 0.021 |
| High; n (%) | 2405 (16.7%) | 974 (27.2%) | <0.001 |
Continuous variables as mean and standard deviation; categorical variables as frequency and percentages; p-value <0.05 taken as statistically significant. Familial Mediterranean fever (FMF); systemic lupus erythematosus (SLE); diabetes mellitus (DM); hypertension (HTN); chronic kidney disease (CKD); cerebrovascular accident (CVA); rheumatoid arthritis (RA); coronary artery disease (CAD); peripheral arterial disease (PAD); venous thromboembolism (VTE).
In bold, p-value <0.05.
Demographics and clinical characteristics of SLE and controls with FMF.
| Variables | Controls with FMF (n=115) | SLE with FMF (n=47) | p-value |
|---|---|---|---|
| Age; years (mean±SD) | 56±15 | 56±13 | 0.904 |
| Females; n (%) | 37 (32.1%) | 16 (34%) | 0.157 |
| Comorbids | |||
| DM; n (%) | 33 (28.6%) | 13 (27.6%) | 0.711 |
| HTN; n (%) | 42 (36.5%) | 17 (36.1%) | 0.873 |
| CKD; n (%) | 5 (4.3%) | 3 (6.3%) | 0.158 |
| CVA; n (%) | 6 (5.2%) | 2 (4.2%) | 0.472 |
| CAD; n (%) | 27 (23.4%) | 12 (25.5%) | 0.079 |
| PAD; n (%) | 7 (6%) | 4 (8.5%) | 0.061 |
| VTE; n (%) | 3 (2.6%) | 3 (6.3%) | 0.041 |
| Thyroid disease; n (%) | 5 (4.3%) | 2 (4.2%) | 0.703 |
| Cancer; n (%) | 1 (0.8%) | None | N/A |
| Socioeconomic status | |||
| Low; n (%) | 74 (64.3%) | 26 (55.2%) | 0.023 |
| Medium; n (%) | 23 (20%) | 12 (25.5%) | 0.036 |
| High; n (%) | 18 (15.6%) | 9 (19.1%) | 0.094 |
Continuous variables as mean and standard deviation; categorical variables as frequency and percentages; p-value <0.05 taken as statistically significant. Familial Mediterranean fever (FMF); systemic lupus erythematosus (SLE); diabetes mellitus (DM); hypertension (HTN); chronic kidney disease (CKD); cerebrovascular accident (CVA); rheumatoid arthritis (RA); coronary artery disease (CAD); peripheral arterial disease (PAD); venous thromboembolism (VTE).
In bold, p-value <0.05.
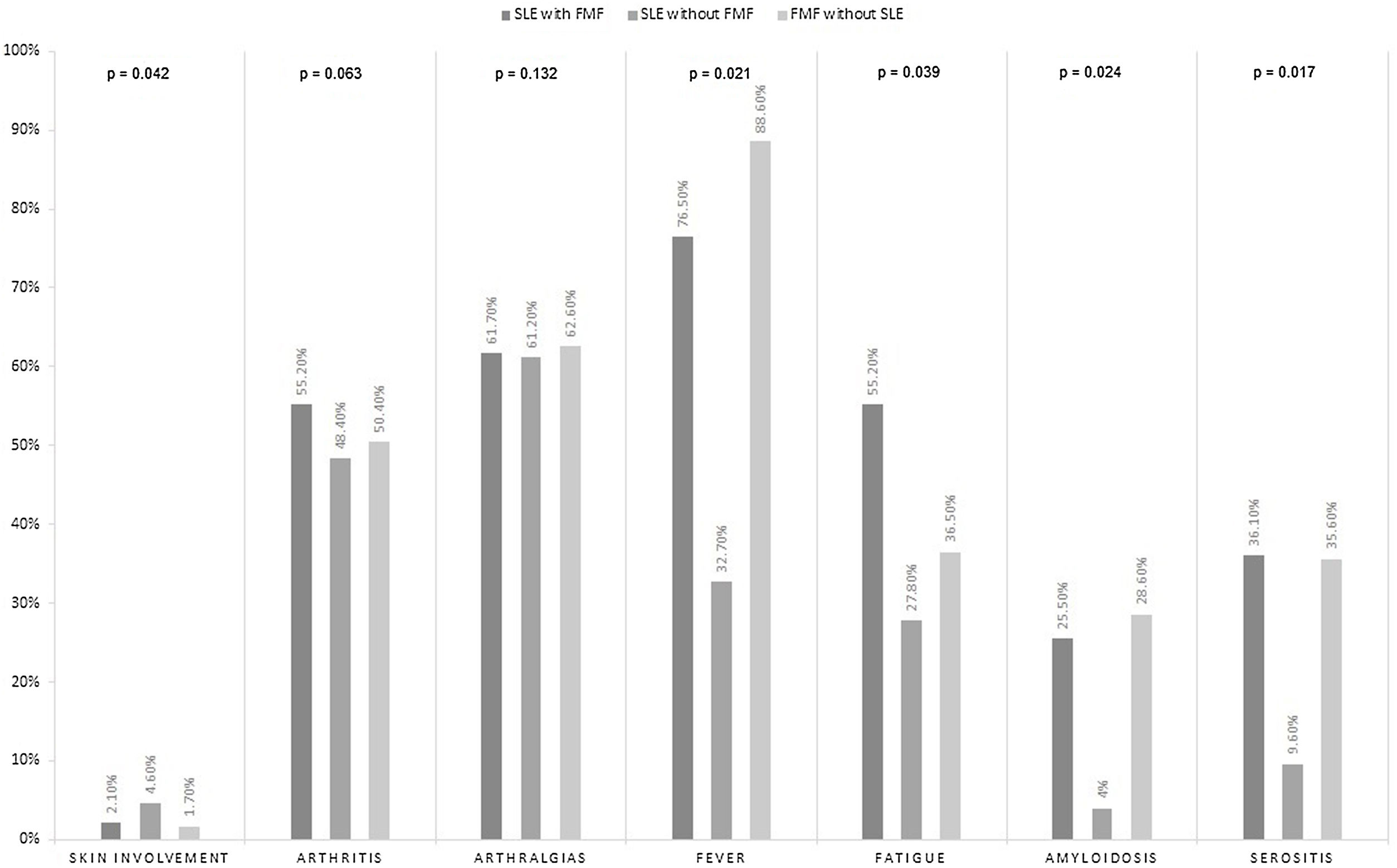
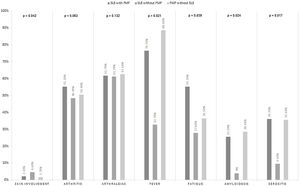
In addition, patients with FMF had a significantly lower socioeconomic group and patients with SLE had medium socioeconomic status (Tables 1 and 2). Disease-related symptoms are summarized in Fig. 2 (Supplementary Table S1). Skin involvement was present in SLE (4.6%; p=0.042) while serositis (35.6%), amyloidosis (28.6%), and fever (88.6%) were more prevalent in FMF. Fig. 3 (Supplementary Table S2) shows various ethnicities in our patient cohort. SLE was more prevalent in Pashtun's (50%) while FMF was dominant in Punjabi's and Sindhi's (53%).
DiscussionThis observational study was designed to investigate the co-occurrence of FMF with SLE. The current evidence from our study demonstrates that patients with SLE have a significantly increased association with FMF when compared to the rate in their matched controls. Patients with SLE have more comorbid conditions related to cardiovascular and cerebrovascular diseases. This is the largest patient cohort in South Asia to be analyzed for this co-existence.
These findings are consistent with one other study conducted in Israel by Klein et al.15 The study population consisted of 4886 SLE patients and 24,430 age and sex-matched controls. Within the SLE group, a significantly higher predominance of FMF patients was demonstrated when compared with controls. However, one case series exhibited a protective effect of SLE by preventing co-existent FMF.16 Their hypothesis states that elevated C-reactive protein (CRP) and amyloid P levels that occur in FMF regularly, may dissipate the formation of autoantigens and the disposal of apoptotic material, leading to a decreasing effect of immune complex-mediated cell injury.17
FMF is an ethnicity-specific disease of the Mediterranean region but our study demonstrated an increased propensity of co-existence of both SLE and FMF in patients of South-Asian origin as well. This is important because FMF is a prevalent disease in Jews and the above-mentioned study by Klein et al. could have inadvertently produced a selection bias with more FMF cases in the general population.15,18 However, the presence of FMF in our patient cohort demonstrates a genetic predisposition of the MEFV mutation with SLE. Its presence can modify the phenotype of the disease by elevating inflammatory attacks and accelerate organ involvement, leading to severe manifestations and mortality.19 In one study, the frequency of MEFV mutation (M6801, V726A, M694V, and E148Q) in a Turkish cohort of Juvenile and adult SLE were 22.7% and 15%, respectively.20 When compared with the healthy Turkish population, the frequency was between 10 and 20%, which is lower than those with SLE.
There is one possible mechanism that can relate to the activation of the inflammatory process in SLE. Cellular apoptosis in FMF can produce intra-nuclear auto-antigens, leading to activation of the adaptive immune cycle by B lymphocytes and increased production of auto-antibodies similar to the classical auto-antibody markers observed in SLE (ANA, anti-ds DNA, anti-Smith antibody).21,22 Autoinflammatory diseases are associated with mutations in the inflammasome-forming NOD-like receptor (NLR), which is a factor responsible for the innate immune system. Interleukin-1 β acts on B and T lymphocytes to amplify their response. This acts as a catalyst for NLR activation and auto-antibody formation. This pathophysiology is described by Doria et al. in her study, in addition to a link between single nucleotide polymorphisms (SNPs) with SLE.23
As compared to the prevalence in the Mediterranean region, FMF is a rare disease in South Asia.24 However, this investigation demonstrates that overlap of symptoms of SLE and FMF can be a reason for the under-diagnosis of FMF in our population cohort. According to Klein et al., there was a considerable overlap of arthralgia, arthritis, and fever.15 The findings of our study showed an overlap of arthralgia and arthritis. Conventional FMF symptoms in patients with concomitant SLE were more prominent than patients without FMF. This demonstrates a diversity of disease and regional variation in the presentation of the two auto-inflammatory diseases.25
Both SLE and FMF are inherited as autosomal recessive traits, and there is a propensity for the transfer of disease through carriers.26,27 Hence, SLE is a disease of low to a medium socioeconomic groups in South Asia, especially in Pakistan where consanguineous marriage is preferred as a social custom. This was demonstrated in our study as FMF and SLE were prevalent in low and medium socioeconomic groups, respectively. A study by Corry et al. suggests that consanguineous marriages result in increased autosomal recessive diseases in the United Kingdom-born Pakistani children, which is associated with the custom of consanguineous marriage.28
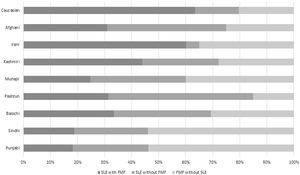
We also investigated the prevalence of both diseases and their co-existence in various ethnicities. SLE was prevalent in every ethnic group with a majority in Pashtuns and Afghanis while FMF alone was prevalent in Punjabis and Sindhis. It was least common in Iranis while on the contrary, SLE with co-existent FMF was common in this ethnic group. Consistent with our findings, the presence of autoinflammatory disorders in the Irani population is 27.4% as carriers and 15.6% as allele frequency.29,30 However, there is a gap between the two results. It was previously believed that SLE and FMF rarely co-exist and this was based on case reports and small sample studies. This investigation is the first to examine the prevalence of FMF and its co-occurrence with SLE. Therefore, it reflects a valid co-occurring pattern between SLE and FMF.
LimitationsWe acknowledge that our investigation had a few limitations. Firstly, there were no genetic tests, lab or pathology parameters available for comparison. Secondly, the study was an institution-based investigation that may not reflect the “real world” analysis of the prevalence of the two diseases in our region. However, it was a large cohort and the first study of this kind from South Asia.
ConclusionIn conclusion, this observational investigation suggests that a higher percentage of SLE is associated with FMF patients when compared with the general population. The current evidence confirms a co-occurrence of auto-inflammatory and autoimmune diseases. Concomitantly, our investigation demonstrates that symptom overlap between FMF and SLE should keep the physicians to consider the alternate diagnosis in patients with recurrent fevers and arthralgias. This study is important to increase awareness of this comorbidity in South Asian countries like Pakistan, where FMF is rare and delayed diagnosis can lead to a delay in initiation of proper treatment.
FundingThe authors received no funding for this manuscript.
Author contributionMUF: concept, data collection, methodology; HS: first draft, final draft; MM: first draft, final draft; RI: first draft, final draft; JM: first draft, final draft, statistical analysis.
Conflict of interestThe authors declare no conflict of interest.