The aim of this study was to determine the prescription pattern and the safety profile of non-steroidal anti-inflammatory drugs (NSAID), including cyclooxygenase-2 inhibitors (COXIB) in patients with rheumatoid arthritis (RA) under a real life clinical setting.
Patients and MethodsData was obtained from the EMECAR study, a prospective cohort of randomly selected RA patients (n=789) followed up from 1999 to 2004 in Spain.
ResultsUpon entry into the cohort, 613 (78%) patients included took or had taken NSAID because of RA. Patients treated with NSAID, compared to those who did not take these compounds, were younger (60 versus 66 years of age) and have had both less cardiovascular (11 versus 20%; p<0.001) and gastric ulcer (11 versus 23%; p<0.001) complications. In the group of patients that used NSAID, RA had been diagnosed earlier (age at onset 47 versus 53; p<0.001) and was more active (DAS28: 4.4 versus 3.7; p<0.001). During follow-up, the percentage of RA patients using NSAID decreased from 78% in year 2000 to 66% in 2004. The use of antiulcer agents increased from 11% in 2000 to 60% in 2004, independently of both the use of classic NSAID or COXIB and the presence of risk factors for NSAID-induced gastropathy. Severe gastric complications and cardiovascular events were infrequent and the incidence was not different between patient who took NSAID and those who did not, as well as between patients treated with classic NSAID or with COXIB.
ConclusionsNSAID are commonly used in the management of RA. These compounds are well tolerated and the frequency of severe adverse events attributed to them is relatively low under daily practice conditions in these patients.
La finalidad de este estudio fue determinar el patrón de prescripción y el perfil de seguridad de los antiinflamatorios no esteroideos (AINE), incluyendo los inhibidores de la ciclooxigenasa-2 (COXIB) en pacientes con artritis reumatoide (AR) bajo condiciones de práctica clínica diaria.
Pacientes y MétodosLos datos fueron obtenidos del estudio EMECAR, una cohorte prospectiva de pacientes seleccionados aleatoriamente (n=789), seguidos desde 1999 hasta 2004 en España.
ResultadosEn el momento de entrar en la cohorte, 613 (78%) pacientes tomaban o habían tomado AINE debido a la AR. Los pacientes tratados con AINE, en comparación con los que no los tomaron, eran más jóvenes (60 contra 66 años) y habían tenido menos complicaciones cardiovasculares (11 contra 20%; p<0.001) y gástricas (11 contra 23%, p<0.001). En el grupo de pacientes que usaron AINE, la AR había sido diagnosticada antes (edad de comienzo 47 contra 53; p<0.001) y estaba más activa (DAS28: 4.4 contra 3.7; p<0.001). Durante el seguimiento, el porcentaje de pacientes con AR que usaban AINE descendió de 78% en el año 2000 hasta el 66% en el 2004. El uso de agentes antiulcerosos se incrementó desde el 11% en el 2000 hasta el 60% en el 2004, de forma independiente al uso de AINE clásicos o de COXIB y de la presencia de factores de riesgo para gastropatía inducida por AINE. Las complicaciones gástricas y cardivasculares severas fueron infrecuentes y su incidencia no fue influida por la toma de AINE o COXIB.
ConclusionesLos AINE son usados muy frecuentemente en los pacientes con AR. Estos compuestos son bien tolerados por estos pacientes y la frecuencia de efectos adversos atribuidos a su uso es relativamente baja en condiciones de práctica clínica diaria.
Rheumatoid arthritis (RA) is a chronic inflammatory disease of unknown etiology and autoimmune nature that usually causes destruction of diarthrodial joints. The most important consequences of RA are pain, physical disability and a reduction in both quality and life expectancy.1 The goal of RA management is to suppress inflammation and prevent structural damage.2 However, as in other chronic diseases, there is an important variability in the use of the pharmacological arsenal for RA in daily clinical practice.3
It is currently accepted that the best way to prevent structural damage in RA is to start treatment with disease-modifying anti-rheumatic drugs (DMARDs) early in the onset of the disease. Although it is well established that non-steroidal anti-inflammatory drugs (NSAID) cannot induce remission or modify the radiological progression of RA,4,5 these compounds are commonly prescribed for this disease because they provide relief of joint pain and stiffness.2
NSAID are a heterogeneous group of chemical compounds commonly prescribed for the symptomatic treatment of a broad variety of pain disorders. The main mechanism of action of NSAID is the inhibition of cyclooxygenase (COX), an enzyme with at least two isoforms, COX-1 and COX-2, essential for the synthesis of prostaglandins. However, several prostaglandin-independent mechanisms of action have also been described for NSAID.6–8 The long-term use of these compounds has been associated with potentially severe side effects, mainly gastrointestinals.9 This is especially relevant in patients under concomitant treatment with steroids or anticoagulants, in those who have a previous clinical history of ulcerous disease and in the elderly.10 Since 1999, selective COX-2 inhibitors (COXIB) have been available in the market. These compounds have a better gastrointestinal safety profile, similar clinical efficacy,11 but higher economic cost than classic NSAID. However, recently two shadows hang over COXIB. They have not proven to be better than the combination of a classic NSAID with a proton pump inhibitor (PPI) in the secondary prevention of bleeding ulcer12 and several studies suggest that COXIB have been more frequently associated with cardiovascular events than classic NSAID.13,14
Whereas, most of the current clinical data concerning NSAID in RA come from clinical trials, very little is known about the pattern of use and safety of these compounds in daily practice. The present work analyzes the use of classic NSAID and COXIB in the EMECAR cohort, a random sample of patients with RA followed-up annually between 1999 and 2004 in Spain.15,16 The main purpose of this study was to determine the pattern of use and the safety profile of these compounds in RA patients in a real life setting.
Patients and methodsStudy designThe data presented herein were obtained from the EMECAR cohort (Estudio de la Morbilidad y Expresión Clínica de la Artritis Reumatoide), a prospectively designed cohort of RA patients promoted by the Spanish Society of Rheumatology in 1999. The main aim of this study was to determine the clinical situation, the management and the progression rate of patients with RA in Spain, as well as to estimate the frequency of both complications and associated comorbidity of this disease.17
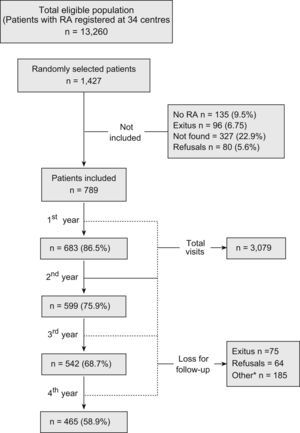
PatientsThe cohort was established by random sampling of patients diagnosed of RA registered in clinical databases of 34 participating centers in Spain. From a total eligible population of 13260 patients, 1427 were randomly chosen and examined to confirm the RA diagnosis according to the ACR 1987 criteria.18 Twenty-five patients per center (a total of 850 patients) were initially selected from each confirmed pool. The rest remained in reserve. Patients not included (135 did not meet RA criteria, 96 had died and 327 were not found) were replaced by the subsequent patients on the selection list (Fig. 1). Eighty patients who did not want to take part in the study were not replaced. The protocol was revised and approved by ethical committees from the centers taking part in the study. Those patients who joined the cohort signed a consent agreement after being informed in detail of the nature and the purpose of the study.
Finally, the baseline visit included 789 patients, 73% of them women and 75% with a positive rheumatoid factor. The mean age at diagnosis was 48±15 years and the mean disease duration at entry was 10±7 years, with 11% of the patients showing a disease evolution of less than two years (Table 1).
Demographic and Baseline Clinical Characteristics of Patients with (NSAID+) and without (NSAID-) NSAID treatment
| NSAID +n=613 (78%) | NSAID –n=176 (22%) | |
| Sex, no of females (percentage) | 450 (73) | 118 (67) |
| Age (years), mean±SD | 60±12* | 66±14 |
| Age at diagnosis of the disease, mean±SD | 47±14* | 53±16 |
| Disease duration (years), mean±SD | 10±8 | 10±8 |
| Rheumatoid Factor + | 454 (75) | 124 (75) |
| Global DMARD treatment | 590 (96)* | 157 (89) |
| Combined therapy (2 or more DMARDs) | 129 (21) | 28 (16) |
| Corticoids (> 3months) | 493 (83) | 132 (81) |
| Antiulcer drugs (PPI and anti H2) | 20 (11) | 68 (11) |
| Comorbidity | ||
| Cardiovascular (total) | 66 (11)* | 35 (20) |
| Congestive heart failure | 28 (5)** | 15 (9) |
| Stroke | 14 (2)* | 15 (9) |
| Ischemic heart disease | 35 (6)* | 16 (10) |
| Arterial hypertension | 216 (35) | 70 (41) |
| Diastolic arterial tension | 80 (11) | 79 (10) |
| Diabetes | 52 (9) | 18 (11) |
| Gastrointestinal ulcer | 65 (11)* | 39 (23) |
| Hypercholesterolemia | 177 (30) | 64 (35) |
| Chronic obstructive pulmonary disease | 45 (7) | 18 (11) |
| Smoker | 211 (35) | 63 (38) |
| Liver disease | 37 (6) | 10 (6) |
| Hip osteonecrosis | 11 (2) | 2 (1) |
| Depression | 111 (18) | 22 (13) |
| Cytopenias | 146 (24) | 38 (23) |
| Osteoporosis | 58 (17) | 14 (15) |
| Bone mineral density in gr/cm2, average±SD | 0.89±0.17 | 0.84±0.18 |
| Osteoporotic fractures | 62 (10)* | 36 (22) |
| Cancer | 20 (3) | 6 (3) |
| Infections | 68 (11)* | 30 (17) |
| RA complications | ||
| Rheumatoid vasculitis | 9 (1) | 1 (1) |
| Serositis | 13 (2) | 4 (2) |
| Felty's syndrome | 1 (0) | 1 (1) |
| Interstitial lung disease | 15 (2) | 7 (4) |
| Sjögren's syndrome | 90 (15) | 28 (17) |
| Eye involvement | 15 (2) | 7 (4) |
| Amyloidosis | 3 (0) | 3 (2) |
| Carpal tunnel syndrome | 67 (11) | 16 (9) |
| Methotrexate pneumonitis | 3 (1) | 3 (2) |
| Disease activity, progression and quality of life | ||
| DAS28, mean±SD | 4.4±1.4* | 3.7±1.4 |
| Remission according to Pinal's criteria | 89 (15)* | 50 (28) |
| HAQ, mean±SD | 1.2±0.8 | 1.3±1 |
| Larsen score, mean±SD | 54±26 | 58±28 |
| SF-12 physical component, mean±SD | 39±10** | 42±12 |
| SF-12 mental component, mean±SD | 47±11* | 51±10 |
DAS28: Disease ActivityScore28joints; DMARDs: disease modifyinganti-rheumatic drugs; PPI: proton pump inhibitors; RA: rheumatoidarthritis; SD: standard deviation.
Results are expressed in absolute numbers of patients and relative frequencies (in brackets), except where indicated.
* p<0,01, ** p<0,05.
Baseline visits of the enrolled patients were carried out between November 1999 and November 2000. Data about the evolution of the disease, complications, comorbidities, and treatments were obtained retrospectively from the clinical records and contrasted with the patients at the start-up visits. The clinical and radiological status of each patient was also recorded. The prospective follow-up of the cohort lasted for 4 years and data acquisition concluded in November 2004. In the results section, we will refer to the visits by years; thus, the basal visits took place in 2000 and the last ones in 2004. Fig. 1 shows the flow of patients throughout the study, as well as the reasons for attrition, which ranged from 7.2% to 13.5% by year.
The rheumatologists involved in the study were instructed in collecting the data and performing the joint counts and other measurements. In all visits, patients were physically examined and laboratory tests were carried out. The functional state and quality of life were assessed through the Spanish versions of the HAQ19 and of the SF-12,20 respectively. Disease activity was determined through the disease activity score obtained from 28 joint counts (DAS28) using the formula with three parameters.21 Hand X-rays were taken at the baseline visit (year 2000), in the third year (2002), and in the last one (2004), and the radiological progression was assessed centralized by an experienced reader using the Larsen method with the Scott modification (range 0–150).22 In addition, treatments with NSAID, corticoids, and DMARDs, as well as their combinations, reasons for withdrawals or substitutions, necessity of intra- and periarticular infiltrations, the use of painkillers, the use of antiulcer drugs (specifically PPI) and other concomitant treatments were registered in every visit. Side effects attributed to NSAID, were also recorded and classified, according to their seriousness, as mild or severe. Mild side effects included headache, diarrhea, dyspepsia, rash, blood hypertension, insomnia, sickness, nausea, tenesmus, and dizziness; severe side effects included perforation, ulcer, and gastrointestinal bleeding, as well as cardiovascular events (congestive heart failure, ischemic heart disease and stroke).
Statistical analysisFor the description of the cohort, means, medians and absolute and relative frequencies were used. Student's t-test was used for comparisons between groups with continuous variables and normal distribution. Discrete variables not normally distributed were analyzed with the Mann–Whitney's U test and categorical variables with the chi-square test. A two-tailed alpha level (p) of 0.001 was agreed for statistical significance, instead of the more common p<0.05 level, to account for multiple comparisons. An estimation of the rate of gastrointestinal hemorrhages and cardiovascular events with 95% confidence intervals was also carried out. The association of factors with NSAID intake during follow-up was measured and tested by means of Cox-proportional hazard models. In a first step, bivariate models were performed, and then multivariate analyses were carried out including only those variables initially found to have a p<0.01 in the bivariate models. Two sets of analyses were carried out: models explaining the use of NSAIDs by variables related to the indication—namely stiffness, CRP, disease activity by DAS28 and VAS of global pain— and models explaining the use of NSAIDs by safety-related variables—age, steroids use, previous ulcer disease, hypertension, hypercholesterolemia, smoking habit, and diabetes—. Data analyses were re-run for COXIB only. We also analyzed the longitudinal risk of gastric ulcer, cardiovascular events, and cancer, associated with NSAIDs, controlling for steroids use, age, previous ulcer disease, and gastric protection, also with Cox-proportional hazard models. All analyses were performed with Stata 9.2 for Windows (StataCorp LP, College Station, TX, USA).
ResultsCross-sectional analysisIn the first visit, 613 (78%) patients confirmed, with no gender-related differences, that they took or had taken NSAID at any time because of RA. A complete description of the demographic characteristics, drugs used, complications, disease activity, and comorbidity of the patients included in the EMECAR cohort is shown in Table 1. Patients who took NSAID for RA were significantly younger, their disease had an earlier onset, and they had less cardiovascular and gastric comorbidity than patients who had never taken NSAID. RA activity and health-related quality of life were worse in patients on NSAID than in patients who had not taken these compounds. The use of DMARDs was higher in patients on NSAID. With regard to antiulcer medications, only 11% of the RA patients, independently of the use of NSAID, had taken these compounds by year 2000. Surprisingly, the group of patients treated with NSAID had accumulated less osteoporotic fractures than patients without NSAID (see Table 1).
In 2000, indomethacin was the most frequently prescribed NSAID in RA patients, followed by diclofenac and naproxen (Table 2). In 1999, COXIB (rofecoxib) started to be available in the market. Thus, very few patients were using these compounds at the end of 2000. In the baseline visit (ending by November 2000), 68 (11%) of patients in the NSAID group reported having had at least one side effect attributable to NSAID, most of which (72%) were minor gastrointestinal effects (Table 3). The frequency of accumulated side effects reported among the most commonly used NSAID was: 15% for naproxen, 12% for diclofenac, 11% for aceclofenac, and 10% for indomethacin.
Proportion of the different NSAID used during the study period, sorted by frequency at first visit
| Visit (year) | |||||
| NSAID | 0 (2000) | 1 (2001) | 2 (2002) | 3 (2003) | 4 (2004) |
| Indomethacin | 32.0 | 26.0 | 25.2 | 22.3 | 23.3 |
| Diclofenac | 14.5 | 13.2 | 12.3 | 11.8 | 12.3 |
| Naproxen | 11.9 | 11.9 | 11.4 | 14.0 | 11.0 |
| Aceclofenac | 11.1 | 8.5 | 8.3 | 8.6 | 10.4 |
| Meloxicam | 8.8 | 7.7 | 7.4 | 8.1 | 7.9 |
| Piroxicam | 5.4 | 4.5 | 4.3 | 4.0 | 3.5 |
| Ibuprofen | 3.3 | 1.8 | 3.1 | 4.0 | 7.9 |
| Flurbiprofen | 2.1 | 1.8 | 1.4 | 1.6 | 1.6 |
| Nabumetone | 2.1 | 1.6 | 1.7 | 2.2 | 0.9 |
| Ketoprofen | 2.0 | 1.8 | 1.7 | 0.8 | 0.9 |
| Nimesulide | 1.6 | 1.4 | 0.7 | 0.3 | 0.0 |
| Rofecoxib | 1.5 | 9.1 | 6.4 | 5.9 | 3.8 |
| Celecoxib | 0.0 | 6.7 | 11.4 | 11.3 | 12.9 |
| Others | 2.5 | 2.4 | 3.1 | 3.5 | 1.6 |
Side effects reported in the group of patients taking NSAID (all NSAID) consider to be related to the drug
| Side effects | Visit 0 | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Total |
| Slight | ||||||
| Rash | 1 | 6 | 1 | 0 | 0 | 8 |
| Vertigo | 3 | 0 | 1 | 0 | 1 | 5 |
| Dizziness | 3 | 0 | 1 | 1 | 0 | 5 |
| Hypertension | 1 | 2 | 1 | 0 | 0 | 4 |
| Diarrhea | 3 | 0 | 0 | 0 | 0 | 3 |
| Headache | 3 | 0 | 0 | 0 | 0 | 3 |
| Pruritus | 0 | 0 | 2 | 0 | 0 | 2 |
| Tinnitus | 0 | 0 | 0 | 0 | 1 | 1 |
| Apprehension | 0 | 0 | 1 | 0 | 0 | 1 |
| Dyslipidemia | 0 | 1 | 0 | 0 | 0 | 1 |
| Alopecia | 0 | 1 | 0 | 0 | 0 | 1 |
| Insomnia | 1 | 0 | 0 | 0 | 0 | 1 |
| Creatinine increase | 0 | 0 | 1 | 0 | 0 | 1 |
| Acute renal failure | 0 | 0 | 1 | 0 | 0 | 1 |
| Thrombopenia | 1 | 0 | 0 | 0 | 0 | 1 |
| Slight gastrointestinal | ||||||
| Epigastralgia | 19 | 6 | 2 | 4 | 3 | 34 |
| Dyspepsia | 14 | 6 | 2 | 6 | 1 | 29 |
| Pyrosis | 10 | 3 | 2 | 0 | 0 | 15 |
| Nausea | 2 | 1 | 0 | 0 | 0 | 3 |
| Heartburn | 2 | 1 | 0 | 0 | 0 | 3 |
| Gastropathy | 1 | 0 | 0 | 1 | 1 | 3 |
| Meteorism | 0 | 1 | 0 | 0 | 0 | 1 |
| Tenesmus | 1 | 0 | 0 | 0 | 0 | 1 |
| Severe gastrointestinal | ||||||
| Digestive Hemorrhage | 1 | 1 | 0 | 0 | 0 | 2 |
| Rectal Bleeding | 1 | 0 | 0 | 1 | 0 | 2 |
| Hypertransaminemia | 1 | 1 | 0 | 0 | 0 | 2 |
| Gastroduodenal ulcer | 0 | 1 | 0 | 0 | 0 | 1 |
| Cardiovascular | 0 | 10 | 9 | 10 | 12 | 41 |
| Cancer | 1 | 5 | 5 | 4 | 4 | 19 |
| TOTAL | 68 (11%) | 46 (8%) | 29 (6%) | 27 (7%) | 23 (8%) | 194 |
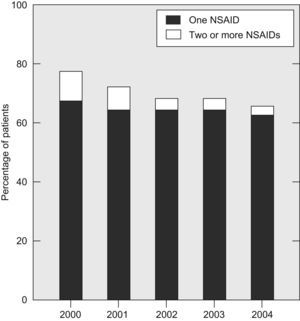
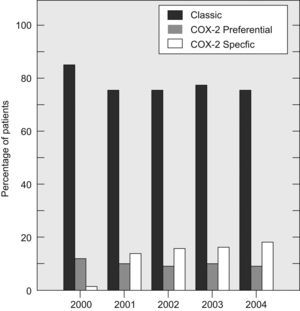
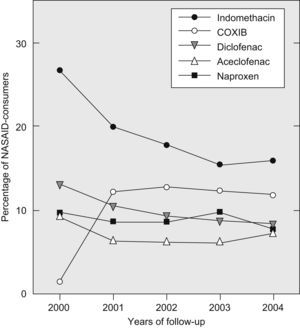
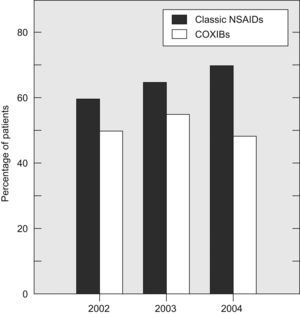
There was a slight, but gradual decline in the percentage of patients with RA using NSAID during the follow-up period (from 78% in 2000 to 66% in 2004) (Fig. 2). Most patients in the NSAID group used a single NSAID throughout the study and only a small percentage of them tried two or more of these compounds consecutively (Fig. 2). The majority of patients who took NSAID used a classic one (Fig. 3). In the year 2000, the percentage of RA patients using COXIB was very low (1.5%). One year later, in 2001, 15.8% of the patients were already using these compounds. Since then, the percentage of COXIB-consumers remained stable along the observational period and at the end of 2004, almost 17% of patients were taking rofecoxib or celecoxib (Figs. 3 and 4). Throughout the study, only 8 patients replaced a COXIB by a classic NSAID. As shown in Fig. 4, indomethacin was the NSAID which experienced the largest decline in consumption, from 32% in 2000 to 23% in 2004.
The use of NSAID (all NSAID) was related to the presence of pain (hazard ratio 1.16, 95%CI: 1.06–1.26; p<0.001), but not to the duration of morning stiffness or to disease activity (DAS28). Conversely, COXIB were preferentially used in patients with high levels of DAS28 (hazard ratio 1.27, 95%CI: 1.14–1.41; p<0.0001). With respect to safety, the presence of risk factors for NSAID-induced gastropathy (all NSAID) was not apparently taken into account by physicians, except in the group of patients older than 65 years (hazard ratio: 0.83, 95%CI: 0.75–0.92; p<0.001). When cardiovascular risk factors were analyzed, only the presence of hypercholesterolemia significantly reduced the use of NSAID (hazard ratio: 0.88: 95%CI: 0.79–0.98; p=0.02). The simultaneous use of antiulcer drugs and NSAID increased during follow-up, from 11% at the initial visit to 75% by the end of the study (Fig. 5). Surprisingly, patients treated with COXIB used antiulcer medications at a similar percentage as patients who were taking classic NSAID (Fig. 5).
The frequency and type of side effects reported to be associated with NSAID consumption during the study are shown in Table 3. Frequencies varied from 11% in the initial cross-sectional assessment—that actually reflects the accumulated rate of side-effects up to first visit—to an annual average of 7% in the longitudinal phase. During the follow-up period, 21 gastroduodenal ulcers, with or without bleeding, were detected. The hazard ratio of using NSAID for presenting an ulcer was 0.88 (95%CI: 0.36–2.19; p>0.05), without adjusting for other variables. When antiulcer drugs were included in the model, the risk of gastroduodenal ulcer did not change substantially (hazard ratio: 1.49, 95%CI: 0.58–4.35; p>0.05). The only independent risk factor for gastroduodenal ulcer was the presence of a history of previous ulcer disease (hazard ratio: 31.36, 95%CI: 9.04–108.74; p<0.0001). The use of NSAID, with or without antiulcer medications did not modify the association with this important risk factor.
Throughout the study, there were 65 cardiovascular events (ischemic heart disease, stroke and congestive heart failure) with an incidence rate of 2.63 (95%CI: 2.06–3.35) per 100 patient∗years. In the group of patients without NSAID, with classic NSAID and with COXIB, the incidence rate of this complication was 3.2 (95%CI: 2.2–4.8), 2 (95%CI: 1.4–2.9) and 4 (95% CI: 2.3–7.1) per 100 patients∗years, respectively. There was no association between NSAID intake (classical and COXIB) and cardiovascular events (hazard ratio: 0.78, 95%CI: 0.47–1.30). The only variables associated with cardiovascular event were age (hazard ratio: 1.04, 95%CI: 1.02–1.07; p<0.0001) and hypertension (hazard ratio: 2.49, 95%CI: 1.68–3.71; p<0.0001). With regard to cancer, a rate of 12.3 per 1000 patient*years (95%CI: 6.4–23.6) was observed in patients without NSAID, 11.9 (95CI%: 7.4–19.2) in those who used classic NSAID and 3.4 (95%CI: 0.4–24.1) in COXIB-treated patients. The only independent risk factor for cancer was age (hazard ratio: 1.04, 95%CI: 1–1.07; p<0.01).
DiscussionNSAID have been successfully used for the symptomatic treatment of both acute and chronic inflammatory disorders.23,24 Regarding RA, NSAID have been profusely used,25 despite ambiguous response to questions such as: “What type of NSAID is more effective in RA?”, “How long must the treatment be maintained?”, or “What are their effects on the functional progression of patients with RA?”.
Upon entry, almost 80% of the patients with RA in our cohort were or had been on an NSAID, at least once during the course of their disease. These compounds were mainly prescribed in patients with a more active disease and seemed to have been avoided in older patients and in those with comorbidities, particularly those who had a history of gastroduodenal ulcer, which reflects an adequate practice in general. In the longitudinal study of the cohort, a slow but significant decrease in the proportion of patients using NSAID was observed, a fact likely to be related to a better control of the disease as a consequence of a more aggressive physician attitude and of the availability of new therapeutic agents (I.G-A. and L.C. unpublished observation). Despite improvement in the control of RA, a high percentage of patients were still using NSAID at the end of the follow-up period of our study. It must be taken into account that the EMECAR cohort followed a RA population with a mean disease evolution of up to 10 years. Consequently, the presence of structural damage in weight-bearing joints may be the reason for a high consumption of NSAID independently of RA activity.
In our cohort, indomethacin, diclofenac, and naproxen represented nearly 60% of the total of NSAID prescribed for RA, being difficult to determine the reason for those preferences. This trend is unlikely to be the consequence of specific marketing strategies, as the three compounds have been in the market for a long time and at relatively low price. We believe that a combination of learnt habits, personal experience and a classic association of those three compounds with greater pain relief in RA patients26 could be the reason for their preferential use in these disease. During the four-year follow-up period, classic NSAID were the most frequently used ones in our series. However, since 2000, NSAID showed a tendency to be less prescribed in favor of COXIB. The treatment with COX-2 preferential inhibitors remained stable at 14% thereafter. On the other hand, the general use of antiulcer agents in combination with COXIB was remarkable. This might be due to either the physicians’ low confidence in the real capability of COXIB to prevent digestive bleeding or to the fact that these drugs produce dyspepsia in a similar proportion as classic NSAID do.
Concerning cardiovascular risk, the link between arterial hypertension and the use of NSAID has been well documented in metaanalysis of randomized controlled trials.27 At the initial visit, 13, 12 and 15% of the patients presented congestive heart failure, stroke, and ischemic heart disease, respectively. These data support a large prevalence of cardiovascular disease in patients with RA.28 During the longitudinal study, we found a tendency to an increased rate of cardiovascular events in patients taking COXIB respect to patients on NSAID, but statistical significance was not achieved, probably due to the low frequency of these events in our series. The incidence of cancer did not differ between patients who took or did not take NSAID. Remarkably, we observed a higher frequency of osteoporotic fractures in patients who did not take NSAID. This data is more likely due to the superior mean age of this group of patients in our cohort than to a potential bone-protective effect of NSAID.
The overall frequency of side effects was less than 5%, most of them mild, which indicates that NSAID are relatively secure compounds in patients without risk factors for gastrointestinal or renal disease. Although there is evidence of less gastrointestinal toxicity of COXIB compared to classic NSAID,12,29,30 we have found that the use of antiulcer agents during the follow-up of our patients was high and did not differ between patients taking NSAID or COXIB. This demonstrates that the current recommendations for gastroprotection in patients treated with NSAID10,31 are not generally followed by rheumatologists in Spain, except in the case of patients older than 65. Consequently, the systematic prescription of PPI, especially in combination with COXIB, causes an increase in pharmaceutical expenses.
The information registered in the EMECAR study shows that NSAID are often used in the management of patients with RA, especially in their more active forms (COXIB) and with higher pain (classic-NSAID). The frequency of adverse effects attributed to these compounds in RA under real life setting is relatively low.
SupportThe EMECAR study was funded by the Spanish Society of Rheumatology and by the Spanish Foundation of Rheumatology. F.D-G. and I. G-A. were recipients of a grant for Research Intensification from Fondo de Investigaciones Sanitarias del Instituto de Salud Carlos III. F. D-G. was supported by FIS 060827 from Instituto de Salud Carlos III.
Conflict of interestsThe EMECAR study was funded by an independent research grant from Aventis.
EMECAR Study Group (in alphabetical order):
Abasolo Alcazar L, Hospital Clínico Universitario San Carlos
Alegre de Miguel C, Hospital de Malalties Reumatiques
Andreu Sánchez JL, Clínica Puerta de Hierro
Aragón Díez A, Hospital Nuestra Señora del Prado
Balsa Criado A, Hospital La Paz
Batlle Gualda E, Hospital General Universitario de Alicante
Belmonte Serrano MA, Hospital General de Castellón
Beltrán Audera J, Hospital Clínico Universitario de Zaragoza
Beltrán Fabregat J, Hospital General de Castellón
Bonilla Hernan G, Hospital La Paz
Carmona Ortells L, Fundación Española de Reumatología
Caro Fernández N, Hospital Nuestra Señora del Prado
Casado E, Hospital Universitario Germans Trias i Pujol
Cebrian Mendez L, Hospital Gregorio Marañón
Corteguera Coro M, Hospital Nuestra Señora de Sonsoles
Cuadra Díaz JL, Hospital Nuestra Señora del Carmen
Cuesta E, Hospital Virgen de La Luz
Fiter Aresté J, Hospital Son Dureta
Freire Gonzalez M, Hospital Gregorio Marañón
Galindo Izquierdo M, Hospital 12 de Octubre
García Meijide JA, Hospital Clínico Universitario de Santiago
García Gómez MC, Hospital de Bellvitge Princeps D’Espanya
Giménez Ubeda E, Hospital Clínico Universitario de Zaragoza
Gómez Centeno E, Hospital Clinic i Provincial
Gómez Vaquero C, Hospital de Bellvitge Princeps D’Espanya
González Fernández MJ, Hospital de Malalties Reumatiques
González Gómez ML, Hospital Gregorio Marañón
González Hernández T, Instituto Provincial de Rehabilitación
González-Alvaro I, Hospital de la Princesa
González-Montagut Gómez C, Hospital Virgen de La Luz
Grandal Delgado Y, Hospital General de Jerez de La Frontera
Gratacos Masmitja J, Complejo Hospitalario del Parc Tauli
Hernández del Río A, Hospital Juan Canalejo
Instxaurbe AR, Hospital de Basurto
Irigoyen Oyarzabal MV, Hospital General Carlos Haya
Jiménez Palop M, Hospital Nuestra Señora de Sonsoles
Juan Mas A, Hospital Son Dureta
Judez Navarro E, Hospital Clínico Universitario San Carlos
Larrosa Padro M, Complejo Hospitalario del Parc Tauli
López Longo FJ, Hospital Gregorio Marañón
Loza Santamaria E, Hospital Clínico Universitario San Carlos
Maese Manzano J, Fundación Española de Reumatología
Manero Ruiz FJ, Hospital Clínico Universitario de Zaragoza
Mateo Bernardo I, Hospital 12 de Octubre
Mayordomo González L, Hospital Universitario de Valme
Mazzucheli R, Hospital Fundación Alcorcón
Medrano San Idelfonso M, Hospital Clínico Universitario de Zaragoza
Naranjo Hernández A, Hospital de Gran Canaria Dr. Negrín
Pecondón Español A, Hospital Clínico Universitario de Zaragoza
Peiró Callizo E, Hospital Virgen de La Luz
Quirós Donate J, Hospital Fundación Alcorcón
Ramos López P, Hospital Príncipe de Asturias
Rivera Redondo J, Instituto Provincial de Rehabilitación
Rodríguez Gómez M, Complejo Hospitalario Cristal-Piñor
Rodríguez López M, Hospital Arquitecto Marcide
Roselló Pardo R, Hospital General San Jorge
Sampedro Alvarez J, Hospital Virgen de La Salud
Sanmartí Sala R, Hospital Clinic i Provincial
Santos Rey Rey J, Hospital Virgen de La Salud
Tena Marsá X, Hospital Universitario Germans Trias i Pujol
Tenorio Martín M, Hospital del Insalud-Ceuta
Torres Martín MC, Hospital Nuestra Señora de Sonsoles
Ureña Garnica I, Hospital General Carlos Haya
Valdazo de Diego JP, Hospital General Virgen de La Concha
Valls M, Hospital Universitario Germans Trias i Pujol
Villaverde García V, Hospital La Paz
Zarco Montejo P, Hospital Fundación Alcorcón
Zubieta Tabernero J, Hospital Virgen de La Salud