Older people with rheumatic diseases tend to have a greater number of associated comorbidities, which will require the use of more drugs, increasing the risk of hospitalizations, complications, and drug interactions. In Mexico, there has been an estimated prevalence of polypharmacy of up to 55%, however there are scarce reports on the topic in our elderly population with rheumatic diseases. We aimed to determine the prevalence of polypharmacy and the association of drug interactions in patients treated for rheumatic disease.
MethodsA retrospective observational study was conducted on patients undergoing treatment for rheumatic diseases who were treated in geriatrics and rheumatology clinics from January to December 2021. The presence of polypharmacy and drug interactions was evaluated using the BOT Plus Pharmacological Surveillance System. The prevalence of polypharmacy and the association of drug interactions were estimated.
ResultsWe evaluated 320 patients, with a mean age of 67.05±5.8 years, predominantly female (85%). The prevalence of polypharmacy was 68.1% (n=218), of which 214 (98.1%) patients had related drug interactions; 27.1% were severe and 53.2% as moderate interactions. Factors related with increased risk of drug interactions were being exposed to hypertension increased the risk of drug interactions (POR 1.75, 95% CI 1.44–2.14; P<0.001), having osteoarthritis (POR 1.21, 95% CI 1.04–1.42; P=0.032) and thyroid disease (POR 1.45, 95% CI 1.28–1.65; P=0.001). The most prevalent serious interactions were leflunomide–methotrexate in 27 (46.5%) patients and buprenorphine–tramadol in 8 (13.7%).
ConclusionsA high prevalence of polypharmacy and drug interactions was observed in elderly patients with rheumatic diseases. The main associated factors were comorbidities, particularly high blood pressure, osteoarthritis and thyroid diseases.
Las personas mayores con enfermedades reumáticas suelen presentar un mayor número de comorbilidades asociadas, que requerirán la utilización de más fármacos, aumentando el riesgo de hospitalizaciones, complicaciones e interacciones medicamentosas. En México se estima una prevalencia de polifarmacia de hasta un 55%; no obstante, existe escasa evidencia en nuestra población mayor con enfermedades reumáticas. Nuestro objetivo es establecer la prevalencia de polifarmacia y el riesgo de interacción medicamentosa en pacientes en tratamiento por enfermedad reumatoidea.
MétodosEstudio observacional y retrospectivo en pacientes en tratamiento de enfermedad reumatoidea que fueron atendidos en los servicios de geriatría y reumatología durante el periodo enero-diciembre de 2021; se evaluó la presencia de polifarmacia e interacciones medicamentosas utilizando el sistema de vigilancia farmacológica BOTplus; se estimó la prevalencia de polifarmacia y el riesgo de interacción medicamentosa.
ResultadosSe evaluó a 320 pacientes, con una media en edad de 67,05±5,83 años, predominando el género femenino en un 85%. La prevalencia de polifarmacia fue del 68,13% (n=218), de los cuales 214 (98,16%) pacientes presentaban interacciones medicamentosas: 58 fueron graves (27,1%) y 114 moderadas (53,12%); se encontró que el padecer hipertensión arterial sistémica aumenta 1,75 veces el riesgo de interacción medicamentosa (OR 1,75, IC95% 1,44-2,14, p=0,000). Las interacciones graves más prevalentes fueron leflunomida-metotrexato en 27 pacientes (46,55%) y buprenorfina-tramadol en 8 pacientes (13,79%). Así mismo, el presentar osteoartritis (OR 1,21, IC95% 1,04-1,42, p=0,032) y enfermedad tiroidea (OR 1,45, IC95% 1,28-1,65, p=0,001) aumentaron significativamente el riesgo de interacción medicamentosa.
ConclusionesSe observó una prevalencia elevada de polifarmacia e interacciones medicamentosas en personas mayores con enfermedades reumáticas. Los principales factores asociados fueron el tener comorbilidades, en particular hipertensión arterial sistémica, osteoartritis y enfermedades tiroideas.
Polypharmacy is defined by the World Health Organization as the concurrent use of three or more drugs. It is categorized as minor when involving 2–4 drugs and major when exceeding five drugs.1 The prevalence of polypharmacy and occurrence of drug interactions in older adults varies and has been estimated to range between 18.2% and 79.5%. In Mexico, approximately 81% of older individuals take medications, with up to 30% taking more than three drugs, a number that increases with age.2
The presence of complex comorbidities often requires the use of multiple drugs to achieve stability and pain control, further complicating the management of polypharmacy. Drug interactions can have diverse effects, such as synergy or antagonism, potentially hindering the clinical progression of the disease and either increasing adverse effects or reducing the intended therapeutic effect.3 Recent studies have shown a direct relationship between the number of drugs taken and the risk of interactions. For instance, consuming five drugs results in a 50% chance of experiencing a clinically significant interaction, which increases to 100% when taking seven drugs. Also, severe interactions were observed in only 20% of cases.4
Drug interactions encompass variations in the action or outcome of a drug, both qualitatively and quantitatively.5 The most common type of drug interaction is drug–drug interaction, which occurs when a drug is added or withdrawn.6 The spectrum of interactions can range from those with no clinical significance to severe interactions that jeopardize the patient's well-being.7
There are several available tools to assess potential drug interactions, including computer systems such as OMI-AP, IDOCTUS, and BOT Plus.8 The BOT Plus platform (https://botplusweb.farmaceuticos.com), developed by the General Council of Official Colleges of Pharmacists (CGCOF), is particularly valuable for estimating the risk of adverse reactions and interactions. By inputting the drugs administered to a patient, the platform provides information on the associated risks.9
A study conducted by Arroyo et al.9 found interactions in 52% of the participants, with an average of 1.79 interactions per person. Around 56% of the interactions were related to long-term therapeutic use medications.10 Rheumatic disease patients are estimated to have a polypharmacy rate of approximately 38–42%, suggesting a high risk of drug interactions.11 Common drugs involved in these interactions include NSAIDs, sulfasalazine, methotrexate, allopurinol, cyclophosphamide, TNF inhibitors, and corticosteroids.12
While the risk of undesirable drug effects appears to increase in patients with rheumatic diseases due to the number of drugs required for their management, the incidence of polypharmacy and the risk of drug interaction in the elderly population remains poorly known. Therefore, we aimed to determine the prevalence of polypharmacy and the risk of drug interactions in older adults undergoing treatment for rheumatic disease.
MethodsWe conducted an observational retrospective study at the geriatrics and rheumatology outpatient clinics at the Hospital General de Zona No. 17 of the Mexican Institute of Social Security (IMSS) in Monterrey, Mexico. This study was performed from January to December 2021. The study was approved by the Local Ethics Committee (approval number: R-2022-1904-151). We included adults aged 65 years or older with a history of rheumatic disease under treatment, polypharmacy, and drug interactions. Non-probability sampling was used, specifically convenience sampling, and in consecutive cases were included, resulting in a total of 362 participants.
Polypharmacy was defined as the simultaneous use of three or more drugs. It was further categorized as minor when involving two to four drugs and major when exceeding five drugs. Drug interaction was defined as variations in the action or outcome of a drug, both qualitatively and quantitatively, and it was estimated using BOT-plus software. The severity of drug interactions was classified according to the Pharmacology Department of the Huddinge Hospital in Stockholm, Sweden. Four groups were classified, ranging from interactions without clinical relevance to severe interactions (A–D). We also collected demographic data and comorbidities of each patient from their clinical history.
We employed the Microsoft Excel 2019 for data collection, and statistical analysis was performed under IBM Stata MP14. Variable normality distribution was evaluated through the Kolmogorov–Smirnov test. Categorical data were reported as proportions and frequencies, and quantitative variables were reported as central tendency (mean, median) and dispersion measures (standard deviation, interquartile range), according to their type of distribution. We compared quantitative variables (e.g., age, number of drugs) with independent samples t or Mann–Whitney tests were used to compare patients with and without polypharmacy, while categorical variables were compared with the Chi-square (X2) test. The confidence level was set at 95% (95% CI) and we considered a P<0.05 as statistically significant.
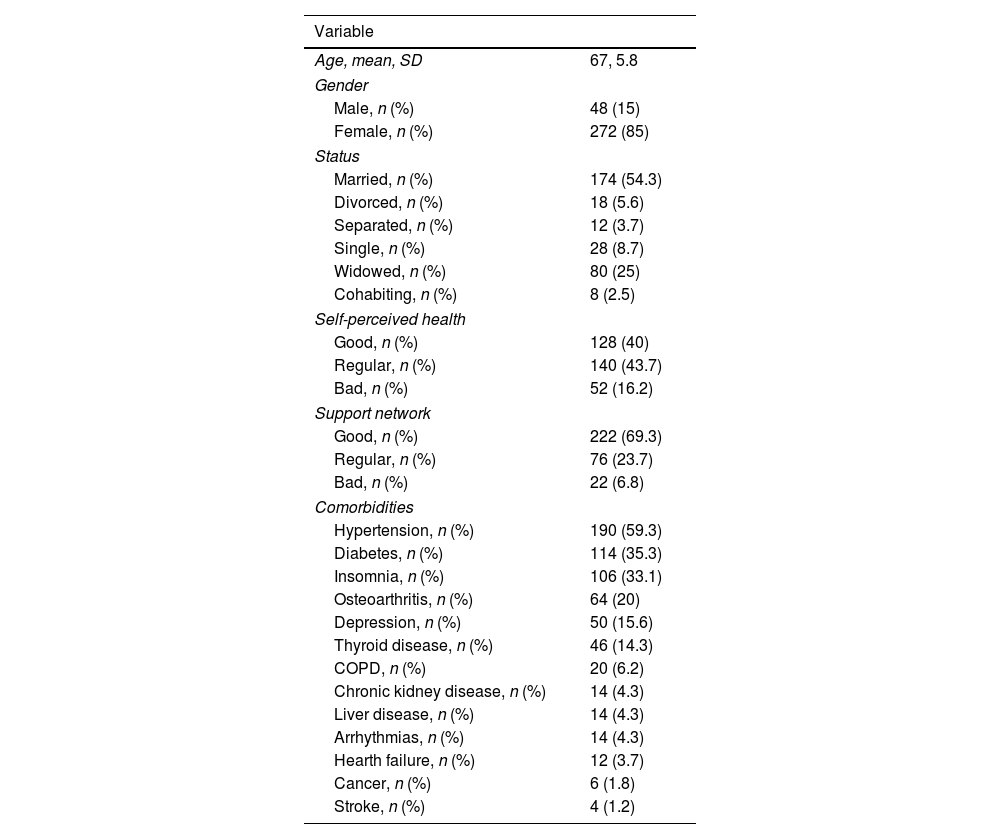
ResultsIn our study, we identified a total of 320 elderly patients with a history of rheumatic diseases who were being treated at our unit. Patients’ mean age was 67±5.8 years, range 55–93; 48 (15%) were men and 272 (85%) were women. Most patients were married (54.3%), considered a regular (43.7%) or good (40%) self-perceived health and had a good perceived support network (69.3%). Among these patients, the most frequent comorbidities were hypertension (59.3%), diabetes (35.3%) and insomnia (33.1%) (Table 1).
Demographic characteristics, self-perceived health and support network, and comorbidities of the included patients.
| Variable | |
|---|---|
| Age, mean, SD | 67, 5.8 |
| Gender | |
| Male, n (%) | 48 (15) |
| Female, n (%) | 272 (85) |
| Status | |
| Married, n (%) | 174 (54.3) |
| Divorced, n (%) | 18 (5.6) |
| Separated, n (%) | 12 (3.7) |
| Single, n (%) | 28 (8.7) |
| Widowed, n (%) | 80 (25) |
| Cohabiting, n (%) | 8 (2.5) |
| Self-perceived health | |
| Good, n (%) | 128 (40) |
| Regular, n (%) | 140 (43.7) |
| Bad, n (%) | 52 (16.2) |
| Support network | |
| Good, n (%) | 222 (69.3) |
| Regular, n (%) | 76 (23.7) |
| Bad, n (%) | 22 (6.8) |
| Comorbidities | |
| Hypertension, n (%) | 190 (59.3) |
| Diabetes, n (%) | 114 (35.3) |
| Insomnia, n (%) | 106 (33.1) |
| Osteoarthritis, n (%) | 64 (20) |
| Depression, n (%) | 50 (15.6) |
| Thyroid disease, n (%) | 46 (14.3) |
| COPD, n (%) | 20 (6.2) |
| Chronic kidney disease, n (%) | 14 (4.3) |
| Liver disease, n (%) | 14 (4.3) |
| Arrhythmias, n (%) | 14 (4.3) |
| Hearth failure, n (%) | 12 (3.7) |
| Cancer, n (%) | 6 (1.8) |
| Stroke, n (%) | 4 (1.2) |
COPD=chronic obstructive pulmonary disease; SD=standard deviation.
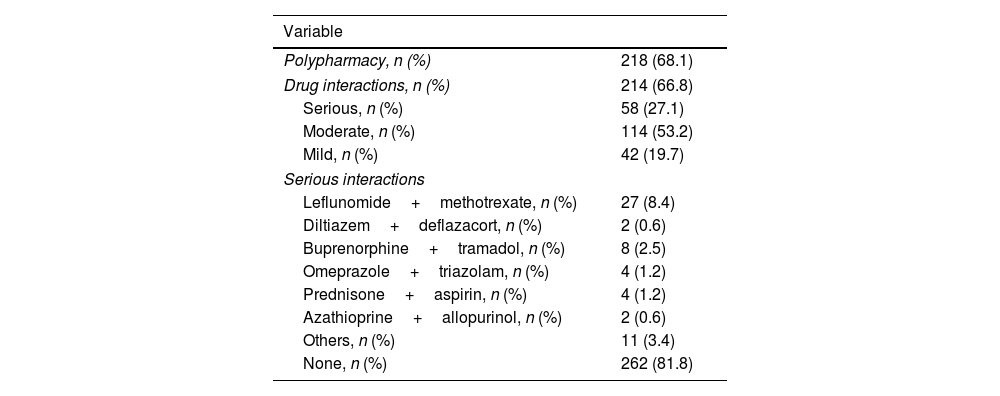
The average number of drugs consumed by the patients was 6.2±3, range 1–16. Polypharmacy was identified in 218 (68.1%) patients. We identified 214 (66.8%) patients with any type of drug interaction. Among these, 58 (27.1%) had severe interactions, 114 (53.2%) had moderate interactions, and 42 (19.7%) had mild interactions. The most frequent serious interaction was leflunomide–methotrexate (27 cases, 46.5%), followed by buprenorphine–tramadol (8 cases, 13.7%), omeprazole–triazolam and prednisone–aspirin (4 cases, 6.8%, each), diltiazem–deflazacort and azathioprine–allopurinol (2 cases, 3.4%, each), and other drug combinations (11 cases, 18.9%) (Table 2).
Polypharmacy and drug interactions identified in the sample.
| Variable | |
|---|---|
| Polypharmacy, n (%) | 218 (68.1) |
| Drug interactions, n (%) | 214 (66.8) |
| Serious, n (%) | 58 (27.1) |
| Moderate, n (%) | 114 (53.2) |
| Mild, n (%) | 42 (19.7) |
| Serious interactions | |
| Leflunomide+methotrexate, n (%) | 27 (8.4) |
| Diltiazem+deflazacort, n (%) | 2 (0.6) |
| Buprenorphine+tramadol, n (%) | 8 (2.5) |
| Omeprazole+triazolam, n (%) | 4 (1.2) |
| Prednisone+aspirin, n (%) | 4 (1.2) |
| Azathioprine+allopurinol, n (%) | 2 (0.6) |
| Others, n (%) | 11 (3.4) |
| None, n (%) | 262 (81.8) |
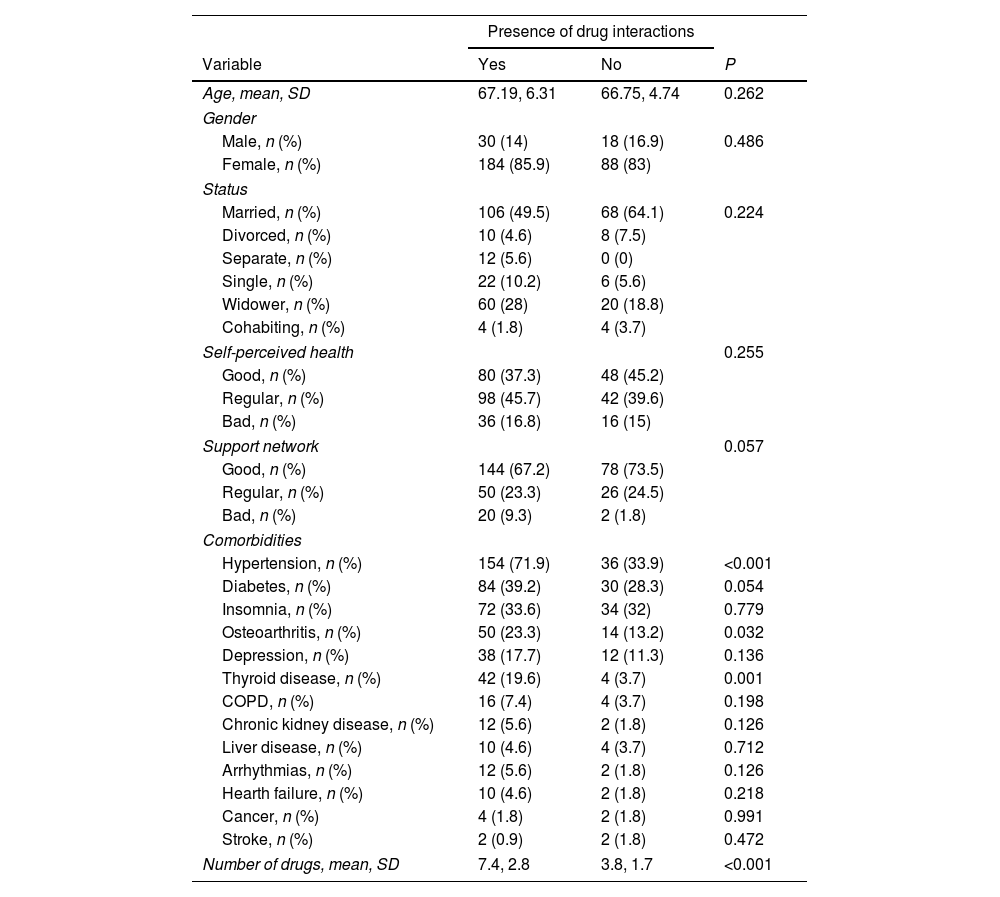
We found no association between the presence of drug interactions and the sociodemographic characteristics, nor the patients’ self-perceived health and perceived support network. We found that patients with drug interactions lived more frequently with hypertension (71.9% vs. 33.9%, P<0.001), osteoarthritis (23.3% vs. 13.2%, P=0.032) and thyroid disease (19.6% vs. 3.7%, P=0.001). As expected, patients with identified drug interactions consumed more drugs (7.4 vs. 3.8 drugs, P<0.001) (Table 3).
Related factors to the presence of drug interactions in the overall sample.
| Presence of drug interactions | |||
|---|---|---|---|
| Variable | Yes | No | P |
| Age, mean, SD | 67.19, 6.31 | 66.75, 4.74 | 0.262 |
| Gender | |||
| Male, n (%) | 30 (14) | 18 (16.9) | 0.486 |
| Female, n (%) | 184 (85.9) | 88 (83) | |
| Status | |||
| Married, n (%) | 106 (49.5) | 68 (64.1) | 0.224 |
| Divorced, n (%) | 10 (4.6) | 8 (7.5) | |
| Separate, n (%) | 12 (5.6) | 0 (0) | |
| Single, n (%) | 22 (10.2) | 6 (5.6) | |
| Widower, n (%) | 60 (28) | 20 (18.8) | |
| Cohabiting, n (%) | 4 (1.8) | 4 (3.7) | |
| Self-perceived health | 0.255 | ||
| Good, n (%) | 80 (37.3) | 48 (45.2) | |
| Regular, n (%) | 98 (45.7) | 42 (39.6) | |
| Bad, n (%) | 36 (16.8) | 16 (15) | |
| Support network | 0.057 | ||
| Good, n (%) | 144 (67.2) | 78 (73.5) | |
| Regular, n (%) | 50 (23.3) | 26 (24.5) | |
| Bad, n (%) | 20 (9.3) | 2 (1.8) | |
| Comorbidities | |||
| Hypertension, n (%) | 154 (71.9) | 36 (33.9) | <0.001 |
| Diabetes, n (%) | 84 (39.2) | 30 (28.3) | 0.054 |
| Insomnia, n (%) | 72 (33.6) | 34 (32) | 0.779 |
| Osteoarthritis, n (%) | 50 (23.3) | 14 (13.2) | 0.032 |
| Depression, n (%) | 38 (17.7) | 12 (11.3) | 0.136 |
| Thyroid disease, n (%) | 42 (19.6) | 4 (3.7) | 0.001 |
| COPD, n (%) | 16 (7.4) | 4 (3.7) | 0.198 |
| Chronic kidney disease, n (%) | 12 (5.6) | 2 (1.8) | 0.126 |
| Liver disease, n (%) | 10 (4.6) | 4 (3.7) | 0.712 |
| Arrhythmias, n (%) | 12 (5.6) | 2 (1.8) | 0.126 |
| Hearth failure, n (%) | 10 (4.6) | 2 (1.8) | 0.218 |
| Cancer, n (%) | 4 (1.8) | 2 (1.8) | 0.991 |
| Stroke, n (%) | 2 (0.9) | 2 (1.8) | 0.472 |
| Number of drugs, mean, SD | 7.4, 2.8 | 3.8, 1.7 | <0.001 |
COPD=chronic obstructive pulmonary disease; SD=standard deviation.
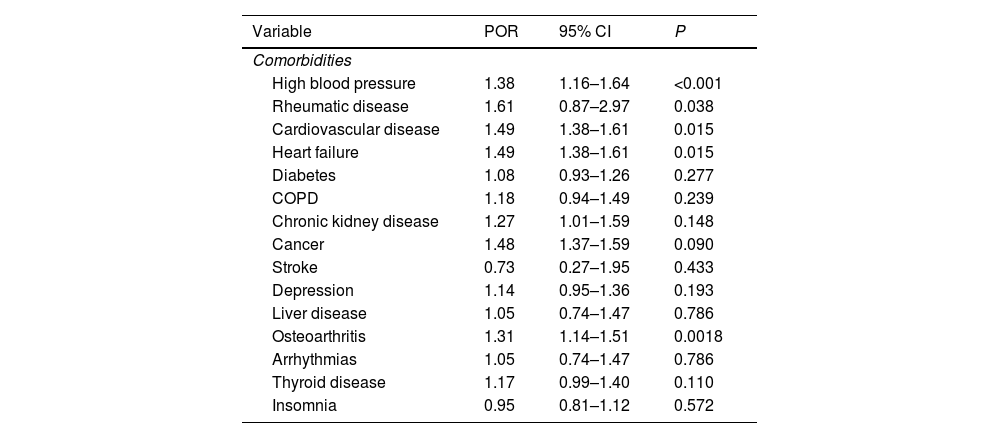
Factors associated with polypharmacy were having high blood pressure (POR 1.38, 95% CI 1.16–1.64; P<0.001), rheumatic disease (POR 1.61, 95% CI 0.87–2.97; P=0.038), cardiovascular disease (POR 1.49, 95% CI 1.38–1.61; P=0.015), heart failure (POR 1.49, 95% CI 1.38–1.61; P=0.015), and osteoporosis (POR 1.31, 95% CI 1.14–1.51; P=0.001) (Table 4). On the other hand, having high blood pressure (POR 1.75, 95% CI 1.44–2.14; P<0.001), osteoarthritis (POR 1.21, 95% CI 1.04–1.42; P=0.032) and thyroid disease (POR 1.45, 95% CI 1.28–1.65; P=0.001) significantly increased the risk of drug interaction.
Associated factors with polypharmacy.
| Variable | POR | 95% CI | P |
|---|---|---|---|
| Comorbidities | |||
| High blood pressure | 1.38 | 1.16–1.64 | <0.001 |
| Rheumatic disease | 1.61 | 0.87–2.97 | 0.038 |
| Cardiovascular disease | 1.49 | 1.38–1.61 | 0.015 |
| Heart failure | 1.49 | 1.38–1.61 | 0.015 |
| Diabetes | 1.08 | 0.93–1.26 | 0.277 |
| COPD | 1.18 | 0.94–1.49 | 0.239 |
| Chronic kidney disease | 1.27 | 1.01–1.59 | 0.148 |
| Cancer | 1.48 | 1.37–1.59 | 0.090 |
| Stroke | 0.73 | 0.27–1.95 | 0.433 |
| Depression | 1.14 | 0.95–1.36 | 0.193 |
| Liver disease | 1.05 | 0.74–1.47 | 0.786 |
| Osteoarthritis | 1.31 | 1.14–1.51 | 0.0018 |
| Arrhythmias | 1.05 | 0.74–1.47 | 0.786 |
| Thyroid disease | 1.17 | 0.99–1.40 | 0.110 |
| Insomnia | 0.95 | 0.81–1.12 | 0.572 |
POR=prevalence odds ratio; CI=confidence interval; COPD=chronic obstructive pulmonary disease.
There are various factors (e.g., age, gender, socioeconomic, cultural, definitions, etc.) that can contribute to variations in the prevalence of polypharmacy and the risk of drug interactions in older adults. Numerous studies have been conducted to determine the prevalence and incidence of polypharmacy and interactions. It has been estimated that 50% of individuals taking five or more drugs simultaneously experience significant interactions, which increases to 100% when seven or more drugs are consumed, leading to severe adverse reactions in 20% of cases.16
Many studies have been conducted to assess the prevalence of polypharmacy across different populations. For example, a study involving over 400 patients with cardiovascular disease found that 84.5% of individuals had polypharmacy, with inadequate prescriptions observed in 48.9% of cases.13 Salech et al.14 reported a prevalence of 65% in elderly adults, while Sanchez et al.15 found polypharmacy in 55% of patients from a Mexican sample aged over 65. Steinman et al.16 however reported a lower prevalence, in 25.8%. Globally, the estimated frequency of potential inappropriate drug use among hospitalized older adults is between 5.8% and 51.4%. In the Mexican population, the prevalence ranges rise from 35% to 65%. In our study, the observed prevalence of polypharmacy was 68.1%, which was higher than expected for our population. This difference may be present as we focused on patients with rheumatic disease, who tend to consume drugs for treatment of their baseline disease and symptom and comorbidity management.
Multiple risk factors are associated with increased polypharmacy. These include frailty, comorbidities, previous hospitalizations, advanced age, and low socioeconomic and educational levels, which may limit the access to health services and contribute to self-prescription and higher rates of polypharmacy. In a study conducted by Gutierrez et al.,17 involving over 7000 individuals, high blood pressure, the use of analgesics and lipid-modifying agents, and the presence of diabetes mellitus were the most commonly associated factors with polypharmacy. Our findings were consistent with their report, as diabetes, thyroid diseases, osteoarthritis, and particularly hypertension were the most frequent factors associated with polypharmacy. Likewise, our study found no association between age and sex, indicating that comorbidities play a more significant role in medication prescribing.
Aging-related physiological changes affect the distribution of medications, including decreased intestinal motility, alterations in absorption, reduction of total body water, decreased muscle mass, and low excretion of drugs due to decreased glomerular filtration rate. These changes influence the distribution, bioavailability, and likelihood of drug interactions in the elderly.18 Thus, the prevalence of drug interactions among elderly outpatients has been studied extensively. The Pharmaceutical Care of the Elderly Europe Research (PEER) study that included 1600 older adults found that 46% of participants had at least one drug combination that could cause a major interaction. Half of these interactions required dose modification to prevent adverse effects.18 A study by Alpizar et al.,6 conducted in 100 elderly Mexican patients, found that 19% had potential drug interactions, with diuretics and NSAIDs being the most common. Kurfees et al.19 reported that 42% of patients in a similar study had potential interactions, with 27% classified as potentially dangerous. Generally, the prevalence of drug interactions in the elderly ranges from 19% to 52%. In our study, the prevalence of drug interactions was 66.8%, which surpasses these previous estimations. Also, the high prevalence of polypharmacy in our sample in patients with potential drug interactions suggests a close relationship between these.
Globally, 4.4% of drug-related hospitalizations are caused by drug interactions. Serious interactions commonly involve methotrexate in combination with NSAIDs or steroids, followed by ipratropium bromide and selective beta-agonists.18 According to a systematic review performed by Hazlewood et al.,20 the combination of methotrexate and immunosuppressants for durations longer than 12 weeks posed a greater risk of adverse reactions. In our study, the most prevalent serious interactions involved methotrexate and leflunomide, in over 12.6% of cases. Methotrexate is commonly prescribed in rheumatic diseases, and its prescription should be accompanied by a careful analysis to prevent potential interactions. A study conducted by G. Nakafero et al.21 developed a tool to stratify the frequency of monitoring blood test during methotrexate treatment.
We identified several limitations of our study. The retrospective nature of this work was limited to the data collected from each patients’ clinical history and we were unable to intervene in cases where polypharmacy or drug interactions were identified at the moment in which this data was analyzed; however, the patients studied remain captive in the rheumatology clinic and are monitored for at least two examinations a year. Also, although these were the drugs documented in the study, we are not certain of other factors that could rise each individual patients’ risk, such as individual drug dose adjustments, treatment adherence, correct administration of drugs, etc. The random selection of participants could give strength to the study; however, the selection method was consecutive based on convenience. We must also disclose that there are limited studies confined to patients with rheumatic disease, making it challenging to compare our findings.
Our study revealed a high prevalence of polypharmacy (over 68%) and drug interactions (66.8%) among older adults with rheumatic diseases. Factors such as comorbidities, particularly arterial hypertension, thyroid disease, and osteoarthritis, were associated with polypharmacy and drug interactions. Further comparative studies involving larger populations are needed to continue analyzing the impact of polypharmacy on older individuals with rheumatological diseases. Also, our findings can help us spread awareness of the problem and most frequent interactions in our patients, and establish a drug conciliation program that could benefit this population.
Authors’ contributionsRLL: Study concept and design, searching the literature, article drafting and critical revision of the manuscript's intellectual content, interpretation of the data, and the decision to submit the article for publication.
DVM: Study concept and design, confirmed the RA diagnosis, and performed the measurements to assess disease activity, article drafting and critical revision of the manuscript's intellectual content, and statistical analysis.
MRSM: Study concept and design, critical revision of the manuscript's intellectual content.
DOB: Revision and preparation of tables, patient recruitment, article drafting, and critical revision of the manuscript's intellectual content.
All authors read and approved the final manuscript.
Consent for publicationNot applicable.
Ethics approvalThe study was approved by the Local Ethics Committee with number R-2022-1904-151.
Availability of data and materialAll data available.
Code availabilityThe authors confirm that the data supporting the findings of this study are available within the article and are available from the corresponding author on reasonable request.
FundingNot apply.
Conflicts of interestThe authors declare that they have no competing interests.
None.