Apremilast is approved for treatment of psoriasis and psoriatic arthritis (PsA). Real-world evidence on apremilast effectiveness in clinical practice is limited.
MethodsObservational study enrolling adult patients, across 21 Spanish centres, who had initiated apremilast in the prior 6 (±1) months and were biologic naive. Data were collected at routine follow-up visits 6 and 12 months after apremilast initiation. Primary outcome was 6 and 12-month persistence to apremilast. Secondary outcomes included Disease Activity for PsA (DAPSA), joint erosions, enthesitis, dactylitis, and patient-reported quality of life (QoL, measured using the PsA impact of disease [PsAID] questionnaire).
ResultsWe included 59 patients. Most had oligoarticular PsA, moderate disease activity, and high comorbidity burden. Three-quarters were continuing apremilast at 6 months and two-thirds at 12 months; mean (SD) apremilast treatment duration was 9.43 (1.75) months. DAPSA scores showed improved disease activity: one-third of patients in remission or low activity at apremilast initiation versus 62% and 78% at 6 and 12 months, respectively. Eleven of 46 patients with radiographic assessments had joint erosions at apremilast initiation and none at month 12. Median (Q1, Q3) number of swollen joints was 4.0 (2.0, 6.0) at apremilast initiation versus 0.0 (0.0, 2.0) at 12 months. Incidence of dactylitis and enthesitis decreased between apremilast initiation (35.6% and 28.8%, respectively) and month 12 (11.6% and 2.4%, respectively). Over two-thirds of patients had a PSAID-9 score <4 (cut-off for patient-acceptable symptom state) at month 12.
ConclusionsIn Spanish clinical practice, two-thirds of PsA patients continued apremilast at 12 months, with clinical benefits at the joint level, no radiographic progression of erosions, and a positive impact on patient-reported QoL.
Trial registration number Clinicaltrials.gov: NCT03828045.
Apremilast está aprobado para el tratamiento de la psoriasis y la artritis psoriásica (APs). La evidencia sobre la efectividad de apremilast en la práctica clínica es limitada.
MétodosEstudio observacional en el que se incluyó a pacientes adultos, de 21 centros españoles, que habían iniciado apremilast en los 6 (± 1) meses previos y no habían recibido biológicos. Los datos se recogieron en visitas rutinarias de seguimiento a los 6 y 12 meses del inicio de apremilast. El objetivo primario fue la persistencia de apremilast a los 6 y 12 meses. Los objetivos secundarios incluyeron la actividad de la enfermedad para APs (DAPSA), erosiones articulares, entesitis, dactilitis y la calidad de vida informada por el paciente (CdV, medida mediante el cuestionario “PsA Impact of disease [PsAID]”).
ResultadosSe incluyó a 59 pacientes. La mayoría presentaba APs oligoarticular, actividad moderada de la enfermedad y alta comorbilidad. Tres cuartas partes continuaban con apremilast a los 6 meses y 2 tercios a los 12 meses; la duración media (DE) del tratamiento con apremilast fue de 9,43 (1,75) meses. Las puntuaciones DAPSA mostraron una mejora de la actividad de la enfermedad: un tercio de los pacientes en remisión o baja actividad al inicio de apremilast frente al 62 y el 78% a los 6 y 12 meses, respectivamente. Once de 46 pacientes con evaluaciones radiográficas presentaban erosiones articulares al inicio de apremilast y ninguno en el mes 12. La mediana (Q1, Q3) del número de articulaciones inflamadas fue de 4,0 (2,0, 6,0) al inicio de apremilast frente a 0,0 (0,0, 2,0) a los 12 meses. La incidencia de dactilitis y la entesitis disminuyeron entre el inicio de apremilast (el 35,6 y el 28,8%, respectivamente) y el mes 12 (el 11,6 y el 2,4%, respectivamente). Más de 2 tercios de los pacientes tenían una puntuación PSAID-9 < 4 (punto de corte del estado sintomático aceptable para el paciente) en el mes 12.
ConclusionesEn la práctica clínica española, 2 tercios de los pacientes con APs continuaron con apremilast a los 12 meses, con beneficios clínicos a nivel articular, sin progresión radiográfica de las erosiones y con un impacto positivo en la CdV reportada por el paciente.
Número de registro del ensayo Clinicaltrials.gov: NCT03828045.
- -
Data on the real-world effectiveness and tolerability of apremilast for the treatment of psoriatic arthritis (PsA) are limited.
- -
Our study describes patients initiating apremilast for the treatment of PsA in Spanish clinical practice and reports apremilast effectiveness and persistence over 12 months.
- -
Two-thirds of patients were continuing apremilast at last 12 months, with improvements in disease activity and a positive impact on patient-reported quality of life.
- -
Apremilast was well tolerated and no new safety signals were identified.
Psoriatic arthritis (PsA) is an inflammatory disease that affects 11–42% of patients with cutaneous psoriasis. PsA is characterized by inflammatory arthritis, enthesitis, dactylitis and spondylitis, and is associated with substantial healthcare costs and impaired health-related quality of life (HRQoL) and work productivity.1–3 Despite the availability of efficacious PsA treatments, not all patients achieve satisfactory disease control and patients unresponsive to first-line methotrexate require treatment with biological agents or target-specific disease-modifying anti-rheumatic drugs (ts-DMARDs).4 Apremilast is a non-biologic oral systemic ts-DMARD approved for the treatment of moderate to severe plaque psoriasis, PsA and mouth ulcers associated with Behçet's disease (BE) that are candidates for systemic treatment.5
In 2016, both the European League Against Rheumatism (EULAR) and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) published updated recommendations for PsA management.2,4 More recently, the Spanish Society of Rheumatology (SER) updated its recommendations. These updates included new therapeutic agents such as apremilast and clearly distinguished apremilast from biological therapies.6 However, they did not define the profile of PsA patients who may benefit from apremilast treatment. Randomized clinical trials (RCTs) showed apremilast is effective in PsA patients naïve to biological agents.7 These RCTs had strict selection criteria, and observed effects may not be representative of clinical practice effects. For example, the PALACE 1, PALACE 2, and PALACE 3 trials excluded patients who had not responded to more than three PsA therapies and patients with other inflammatory comorbidities.8–10 To date, few observational studies have assessed the profile of patients receiving apremilast treatment for PsA in clinical practice.
Although clinical practice guidelines provide some recommendations to assess PsA treatment efficacy, the assessment of treatment response in routine practice is heterogeneous. The number of swollen or tender joints, the presence of enthesitis/dactylitis, C-reactive protein (CRP), or patient-reported pain and disease activity are often used in patient follow-up. Most of these evaluations are included in the Disease Activity index for PsA (DAPSA), a validated tool that classifies disease activity as in remission, low, moderate or high.11,12 While a clinical version of the DAPSA index (cDAPSA) that includes all items except CRP has also been validated, the DAPSA and cDAPSA are not widely used in clinical practice.11
Despite the proven efficacy of apremilast and guideline recommendations, data regarding apremilast use and effectiveness in the real-world setting is limited and few studies have assessed apremilast effectiveness using DAPSA. Our study evaluated the effectiveness and persistence of apremilast treatment for PsA in Spanish clinical practice, and describes the profile of patients initiating treatment with apremilast in Spain.
MethodsStudy design and populationThe observational PREVAIL study enrolled consecutive patients aged≥18 years with PsA (CASPAR criteria)13 who had initiated apremilast during the previous 6 (±1) months and had not previously received biologic treatment (ClinicalTrials.gov Identifier NCT03828045). A diagram with the study design is included in Fig. 1. Patients were recruited across 21 centres in Spain and data collected at routine follow-up visits 6 and 12 months (±1 months) after apremilast initiation. Data at apremilast initiation were collected retrospectively from medical records.
Objectives and variablesThe primary study objective was to estimate apremilast persistence at 6 and 12 months; the primary outcome was the percentage of patients continuing apremilast at 6 and 12 months after initiation.
Secondary objectives were to describe the clinical profile of patients initiating apremilast for the treatment of PsA in clinical practice and evaluate disease activity 6 and 12 months after apremilast initiation. Disease activity was assessed using DAPSA score calculated from the information available in the patient's medical record, presence of enthesitis and dactylitis (yes/no), Physician Global Assessment (PGA) and patient-reported quality of life (QoL). The DAPSA score is the sum of five items: (1) 68-tender joint count (68-TJC), (2) 66-swollen joint count (66-SJC), (3) CRP, (4) the patient's global assessment of disease, and (5) the patient's assessment of pain. A DAPSA score≤4 indicates disease remission and a score>28 indicates high disease activity. Patient characteristics at apremilast initiation were retrospectively extracted from medical records.
Patients were asked to complete the VITACORA-19 and Psoriatic Arthritis Impact of Disease (PsAID) questionnaires at 6 and 12 months.14,15 VITACORA is a validated questionnaire of 19 items (range 0–100; higher values indicate better QoL) and has shown good reliability and high responses rates in the Spanish population.14 The nine-domain version of the validated PsAID was used. Each item is rated on a scale from 0 to 10. The scores for each item are weighted, resulting in a final score between 0 and 10, where higher scores indicate greater impact on patient QoL; PsAID>4 is the cut-off for patient-acceptable symptom state. The version used in this study (the PsAID-9) consists of 9 items, which address pain, fatigue/tiredness, skin problems, work and/or leisure activities, the functional capacity to carry out daily activities, the feeling of discomfort/irritation, difficulty sleeping, coping/coping and anxiety, fear and uncertainty.15
Safety and tolerability were assessed using treatment-emergent adverse events (TEAEs), which were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 24.1. Reasons for apremilast discontinuations were also recorded.
Statistical analysisThis observational study was not designed for statistical comparisons or hypotheses testing. Based on the results in a previous study,16 it was expected that 72.1 of the patients with PsA would continue to be treated after 6 months of treatment. To estimate the 95% CI of an expected ratio of 72.1% and a precision of 10%, at least 78 patients were to be included. Assuming a dropout rate of 20%, the inclusion of 98 patients was planned necessary. All analyses were descriptive and included all enrolled patients meeting the study inclusion criteria. Categorical variables were summarized using frequencies and percentages, with percentages calculated from the number of patients with non-missing data. Continuous variables were summarized using the number of non-missing data points, mean, standard deviation (SD), median and interquartile range (IQR). Only the calculation of the PsAID-9 scores included an imputation method; specifically, if one of the 9 questionnaire items was missing, the mean value was calculated from the non-missing values and included in percentage calculations. All statistical analyses were performed using SAS software (version 9.4).
Ethical statementThe study was approved by the Research Ethics Committee of Hospital Universitario de Puerta de Hierro and was conducted in accordance with the Declaration of Helsinki. All patients included in the study signed an informed consent approved by the Ethics Committee.
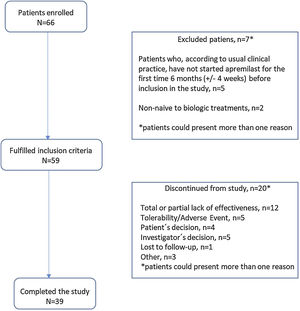
ResultsStudy dispositionOf 66 patients recruited between 6th February 2019 and 20th October 2021, 59 (89.3%) met the study inclusion criteria and were included in the analysis reported herein. Of these, 20 (33.9%) patients discontinued the study before month 12 (Fig. 2); reasons for discontinuation were total/partial lack of effectiveness (12 patients [20.3%]), tolerability/adverse event (5 [8.5%]), investigator decision (5 [8.5%]), patient decision, (4 [6.8%]), lost to follow-up (1 [1.7%]), and “other” (3 [5.1%]). Mean (SD) duration of apremilast treatment was 9.43 (1.75) months for all enrolled patients.
Patient characteristicsPatient characteristics at apremilast initiation are summarized in Table 1. Mean (SD) age was 52.8 (13.5) years; mean (SD) body mass index (BMI), 28.6 (5.7). Most patients (50/59 [85%]) had previously received conventional non-biologic DMARDs, with lack of response and/or lack of tolerance reported in 22/50 (44.0%) and 11/50 patients (22.0%), respectively.
Patient characteristics at apremilast initiation.
| All patients (N=59) | |
|---|---|
| Demography and analytical | |
| Age, mean (SD), years | 52.8 (13.5) |
| Male, n (%) | 31 (52.5%) |
| Weight, mean (SD), kg | 78.9 (15.8) |
| Height, mean (SD), cm | 166.4 (8.7) |
| BMI, mean (SD), kg/m2 | 28.6 (5.8) |
| AST, mean (SD), U/L | 21.0 (7.9) |
| ALT, mean (SD), U/L | 26.1 (17.5) |
| CRP, mean (SD), mg/dL | 1.4 (2.9) |
| Psoriatic arthritis | |
| Time from PsA diagnosis to apremilast initiation, mean (SD), years | 4.4 (5.1) |
| Time from onset of PsA symptoms to apremilast initiation mean (SD), years | 6.1 (4.7) |
| Number of affected joints, mean (SD) | 4.3 (3.7) |
| Number of swollen joints, mean (SD) | 3.1 (3.5) |
| Number of painful joints, mean (SD) | 4.0 (3.7) |
| Dactylitis, n (%)) | 21 (35.6%) |
| Enthesitis, n (%)) | 17 (28.8%) |
| DAPSA, mean (SD) | 20.8 (10.5) |
| Previous PsA treatments | |
| DMARDs, n (%)) | |
| Yesa | 50 (84.7%) |
| Metotrexate | 18 (30.5%) |
| Leflunomide | 44 (74.6%) |
| Sulfasalazine | 7 (11.9%) |
| Comorbidities,n(%)b | |
| Yes | 37 (62.7%) |
| Endocrine/metabolic disease | 20 (33.9%) |
| Cardiovascular disease | 15 (25.4%) |
| Musculoskeletal disease | 9 (15.3%) |
| Other diseases | 9 (15.3%) |
| Respiratory disease | 8 (13.6%) |
| Neoplasms | 8 (13.6%) |
| Psychiatric disease | 6 (10.2%) |
| Allergies | 6 (10.2%) |
| Hepatic disease | 4 (6.8%) |
| Infectious disease | 4 (6.8%) |
| Neurologic disease | 2 (3.4%) |
| Autoimmune disease | 1 (1.7%) |
Data availability for each individual parameter may vary.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C-reactive protein; DAPSA/DAPSAc, Disease Activity in PSoriatic Arthritis; DMARD, disease-modifying anti-rheumatic drugs; PsA, psoriatic arthritis; SD, standard deviation.
The majority of patients (37/59 [62.7%]) had at least one comorbidity, the most common being endocrine/metabolic (20/59 [33.9%]; including obesity, 8/59 [13.5%] and dyslipidemia, 12/59 [20.3%]), cardiovascular (15/59 [25.4%]; mainly hypertension and musculoskeletal diseases 9/59 [15.2%] each), and respiratory diseases (8/59 [13.5%]).
Using the CASPAR criteria,13 most patients had a concomitant diagnosis of psoriasis (51/59 [86.4%]) and negative rheumatoid factor (53/59 [89.8%]). Approximately half had concomitant psoriatic nail dystrophy (29/59 [49.2%]) and over half had dactylitis (32/59 [54.2%]). Among patients with a DAPSA score at apremilast initiation, half (14/28 [50%]) had a score which indicated moderate disease. Overall, 85% (50/59) of patients had peripheral arthritis phenotype. Most patients (50/59 [85%]) had received previous systemic treatment.
PersistenceThree-quarters (45/59 [76%]) of patients were continuing apremilast at 6 months and two-thirds (38/58 [6.55%]) were continuing apremilast at 12 months.
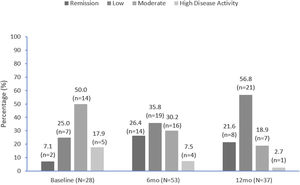
Disease activityDAPSA scores are summarized in Fig. 3 and improved over time. Approximately one-third (9/28 [32.1%]) of patients with DAPSA scores recorded were in remission or had low activity at apremilast initiation, compared with over half (33/53 [62.2%]) at 6 months and over three-quarters (29/37 [78.4%]) at 12 months (Fig. 3). At apremilast initiation mean (SD) DAPSA score values were 20.8 (10.5), 13.7 (10.5) at month 6 and 11.8 (11.4) at month 12. Mean (SD) decrease in DAPSA from apremilast initiation to months 6 and 12 was 7.3 (8.1) and 7.8 (8.2), respectively.
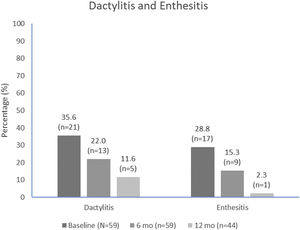
The median (IQR) number of affected joints decreased from 4.0 (2.0, 6.0) at apremilast initiation to 1.0 (0.0, 4.0) at month 6 and 0.0 (0.0, 2.0) at month 12. The proportion of patients with dactylitis and enthesitis also decreased over time (Fig. 4). The most common entheses was the Achilles tendon insertion, reported in 9/17 (52.9%) and 2/8 (22.2%) patients with entheses at apremilast initiation and month 6, respectively.
Radiographic assessments at the time of apremilast initiation were available for most (46/59 [78.0%]) patients, one-quarter (12/46 [26.1%]) had joint erosions and the mean (SD) number of affected joints was 2.7 (1.4). At months 6 and 12, radiological evaluations were performed in 15/59 (25.4%) and 15/43 (34.9%) patients, respectively; 2 (13.3%) patients showed increased erosion at month 6 and no patients showed increased erosion at month 12.
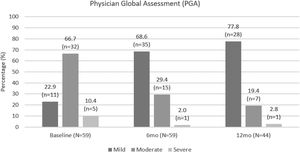
Based on PGA scores, three-quarters of patients had moderate or severe disease at apremilast initiation, compared with only 16/51 (31.4%) of patients at month 6 and 8/36 (22.2%) of patients at month 12. PGA scores were lower at month 6 and month 12 compared with at apremilast initiation. PGA improvements are shown in Fig. 5.
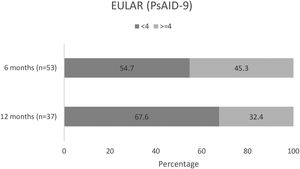
Changes in patient QoLAmong patients completing the PsAID-9 questionnaire, over half (29/53 [54.7%]) had a PsAID-9<4 at month 6 (mean [SD] 3.6 [2.3]), and over two-thirds (25/37 [67.6%]) had a PsAID-9<4 at month 12 (mean [SD] 3.5 [2.4])15 (Fig. 6). In these patients, mean overall PsAID-9 score were lower at months 6 and 12.
Mean (SD) VITACORA-19 scores were 64.0 (17.1) at month 6 and 63.8 (17.0) at month 12.
Safety outcomesTEAEs were reported in 14 patients, the most common being diarrhoea (3 patients, 5.1%), insomnia, and headache (2, 3.4% each). Most TEAEs were temporary and resolved; only one patient discontinued apremilast due to a TEAE (diarrhoea). Only one patient reported symptoms of depressive disorder. Overall, the observed treatment-related TEAEs aligned with the current apremilast safety profile.17
DiscussionWe report real-world evidence (RWE) from Spain on the use of apremilast in patients with moderately active PsA who had not previously received biologics. Most patients included in our study had moderate disease activity, as measured by the DAPSA score, and a high comorbidity burden. In addition to high apremilast persistence, physicians and patients reported good response rates during 6–12 months of apremilast treatment. Specifically, the number of swollen joints, the incidence of dactylitis and enthesitis, and disease severity scores (DAPSA and PGA) all decreased, with the greatest reductions observed at month 12. Two disease-specific instruments (PsAID-9 and VITACORA-19) used to evaluate HRQoL in patients with PsA showed positive outcomes in more than half of the patients.
As expected, the profile of patient's receiving apremilast in Spanish clinical practice differed from that of the pivotal clinical trials and was similar to other RWE studies.18–23 Most patients had long-standing active oligoarticular PsA (defined as <5 involved joints) of moderate severity (mean [SD] baseline DAPSA score, 20.8 [10.5]; mean [SD] time from start of PsA symptoms to apremilast initiation, 6.1 [4.7] years). Moreover, our study population reflected the Spanish Society of Rheumatology recommendations which include apremilast as a treatment option for patients with active PsA whose systemic treatment choices may be limited by efficacy, frequent laboratory safety requirements, and tolerability.6
As in other RWE studies, apremilast was likely prescribed due to co-existing and comorbid diseases, mainly hypertension, dyslipidemia, malignancy and cardiopathy. For example, in patients with oligoarticular PsA and more than 2 comorbidities receiving long-term apremilast treatment. Balato A. et al.20 reported history of malignancy and previous biologic treatment negatively influenced PASI responses. Mazzilli et al.24 and Arias et al.25 reported apremilast to be effective in psoriatic patients affected by cardiometabolic comorbidities. Chan et al.19 reported improved clinical outcomes in an unselected PsA group of patients with multiple co-existing conditions defined as any distinct additional entity that has existed or may occur during the clinical course (malignancy, bronchiectasis, multiple sclerosis) treated with apremilast over one year.
Our study included patients that had received previous conventional systemic therapies but were naïve to biologic agents. Chan et al. reported better response rates in biologic-naïve patients versus subjects with prior bDMARD use with shorter duration of PsA.19 Similarly, the phase IIIB ACTIVE study confirmed long-term apremilast efficacy in biologic-naïve PsA patients with one prior csDMARD according to the American College of Rheumatology 20 criteria (ACR20).7 In an interim analysis of the German LAPIS-PsA study, biologic-naïve patients reported a Patient's Global Assessment of Disease Activity (PtGA) score of 0/1 earlier and more frequently than biologic-experienced patients.22
Several composite measures of disease activity that assess multiple clinical domains have been validated in PsA clinical trials. Our study used the DAPSA score to assess disease activity. Importantly, two of the five DAPSA domains (PtGA and pain) are subjective, patient-reported items and TJC is influenced by a central sensitization of pain.26 The use of composite disease activity measures and response criteria in clinical practice is highly dependent on their feasibility. In this regard, DAPSA defined thresholds can be calculated easily, even in clinical settings with limited time resources.27 In a previous RWE study of patients with oligoarticular PsA (defined as ≤4 swollen joints), those receiving apremilast monotherapy reported larger improvements in cDAPSA score at 6 months than patients receiving methotrexate or bDMARD.20 Notably, in our study, the percentage of patients in remission increased from 7% at apremilast initiation to 22% at 12 months, indicating an overall favourable disease evolution.
PsA treatment aims to achieve the lowest possible level of disease activity and, if possible, remission, minimize joint damage, and gain acceptable physical function and QoL. In our study, patients reported improvements in their QoL 6 and 12 months after apremilast initiation. Moreover, these improvements were observed for both of the questionnaires used (PSAID-9 and VITACORA-19), reflecting a clinically meaningful improvement in QoL. In this regard, several recent studies have shown a good correlation between PSAID and disease activity, and PSAID has been identified as the main factor associated with treatment persistence.28
The 12-month apremilast discontinuation rate of 33.8% observed in our study is consistent with that reported by Balato A. et al. over 52 weeks.21 Among patients discontinuing apremilast, 20.3% stopped due to lack of efficacy, which is in line with the 14–37.5% reported in other RWE studies.21,29 The safety profile of apremilast was aligned with the apremilast SmPC and other RCTs and RWE studies.5,8–10,16,21–24,29–31 AEs were mostly mild (gastrointestinal) and in most cases did not require treatment discontinuations.
Our study has several strengths. The inclusion criteria were broad and we included adult patients with PsA who had initiated apremilast for the first time in the previous 6 months and were biologic-naïve. No other demographic and clinical criteria were considered and we captured the effectiveness of apremilast in real-world populations, supporting the generalizability of the results.
The main limitation of our study is the small sample size, which is, in part, due to the COVID pandemic. During the pandemic, prioritization of COVID-19 research, redeployment of research staff, and the need for social distancing has negatively impacted recruitment to non-COVD related studies. For example, Mirza et al. reported that patients were less willing to participate in observational and interventional rheumatology research studies whilst COVID-19 was present in the community.32 Also, our single-arm study was not designed to make comparisons or test hypothesis, and pain was not systematically assessed at apremilast initiation. While this limits our ability to assess whether symptoms improved over time, PGA values were available and indicated disease severity decreased during apremilast treatment. Despite these limitations, our study captures real-world data of apremilast use, effectiveness, and tolerability.
ConclusionsOur study shows that patients naïve to biologics initiating apremilast for the treatment of PsA in Spanish clinical practice have moderate, non-erosive PsA, and have a high comorbidity burden. Moreover, our data show the clinical benefits of apremilast in these patients, including a reduction in the number of swollen and tender joints, fewer dactylitis and enthesitis, and improved patient quality of life (QoL). Approximately two-thirds of the patients continued on apremilast at 12 months, with no new safety signals.
AuthorshipAll named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Jordi Gratacos-Masmitja, J.C. Torre Alonso and Eva Pascual contributed to the study conception and design. Material preparation, data collection and analysis were performed by all participant authors. The first draft of the manuscript was written by all participant authors and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availabilityQualified researchers may request data from Amgen clinical studies. Complete details are available at the following: https://wwwext.amgen.com/science/clinical-trials/clinicaldata-transparencypractices/clinical-trial-data-sharing-request.
FundingThis study was funded by Celgene. Amgen acquired the worldwide rights to Otezla® (apremilast) on November 21, 2019.
Conflict of interestJordi Gratacos-Masmitja Speakers bureau: MSD, Pfizer, AbbVie, Janssen-Cilag, Novartis, Celgene y Lilly. Consultant of: MSD, Pfizer, AbbVie, Janssen-Cilag, Novartis, Celgene y Lilly. José Luis Álvarez Vega Speakers bureau: Abbvie, Amgen, MSD, Lilly, Roche, Esteve, UCB, Menarini, Pfizer, GSK, BMS, Janssen, Novartis, Gebro. Consultant of: Abbvie, Amgen, MSD, Lilly, Roche, Esteve, UCB, Menarini, Pfizer, GSK, BMS, Janssen, Novartis, Gebro. Grant/research support from: Abbvie, Amgen, MSD, Lilly, Roche, Esteve, UCB, Menarini, Pfizer, GSK, BMS, Janssen, Novartis, Gebro. Emma Beltrán Speakers bureau: Abbvie, Bristol, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer, Roche and UCB. Consultant of: Abbvie, Bristol, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer, Roche and UCB, Ana Urruticoechea-Arana: None declared. C. Fito-Manteca: None declared. Francisco Maceiras: None declared. Joaquin Maria Belzunegui Otano Speakers bureau: Lilly, Amgen, Novartis, Abbvie, Janssen. J. Fernández Melón Speakers bureau: Amgen SL, Eugenio Chamizo Carmona: None declared. Abad Hernández Speakers bureau: MSD, Abbvie, Pfizer, Kern, Novartis, Biogen, Sandoz, Amgen, Sanofi, Lilly, Roche and Janssen-Cilag. Consultant of: MSD, Abbvie, Pfizer, Kern, Novartis, Biogen, Sandoz, Amgen, Sanofi, Lilly, Roche and Janssen-Cilag. Grant/research support from: MSD, Abbvie, Pfizer, Kern, Novartis, Biogen, Sandoz, Amgen, Sanofi, Lilly, Roche and Janssen-Cilag, Inmaculada Ros. Consultant of: Amgen. Grant/research support from: MSD, Roche, Novartis, Lilly, Pfizer, Amgen, Eva Pascual. Shareholder of: Amgen. Employee of: Amgen, Juan Carlos Torre Speakers bureau: Amgen, Lilly, Novartis, Janssen, Pfizer. Consultant of: Amgen, Lilly, Novartis, Janssen, Pfizer. Grant/research support from: Amgen, Lilly, Novartis, Janssen, Pfizer.
The authors would like to thank the patients, medical and nursing staff, and data managers who participated in this study.
The authors thank the i2e3 Procomms team and specially, Marta Ruiz, MD PhD and Sara Cervantes, PhD for providing medical writing support during the preparation of the manuscript; and Bioclever 2005 and specially Josep Puig provided support for data management, statistical analysis, interim and final reports, and editorial assistance writing of the study protocol and this manuscript. Claire Desborough, Amgen Ltd. also provided medical writing support for this manuscript.
Dr. Jordi Gratacós, Dra. Marta Arévalo, Dra. Mireia Moreno, Dra. Emma Beltrán Catalan, Dra. Carolina Pérez García, Dr. Jordi Monfort Faure, Dr. Luciano Polino, Dr. Jose Luis Alvarez, Dra. M. Carmen Carrasco Cubero, Dra. Adela Gallego Flores, Dra. Ana Urruticoechea-Arana, Dra. Concepcion Fito, Dr. Vicente Aldasoro, Dra. Natividad del Val, Dr. Francisco Maceiras, Dr. Rafael Melero, Dr. Nair Pérez, Dr. Joaquín María Belzunegui Otano, Dr. César Antonio Egües Dubuc, Dr. Luis López Domínguez, Dra. Julia Fernández Melón, Dr. Jordi Fiter, Dr. Eugenio Chamizo Carmona, Dr. Raúl Veroz González, Dr. Juan Jose Aznar Sánchez, Dra. Sara María Rojas Herrera, Dr. Miguel Ángel Abad Hernández, Dr. Fernando G. Ruiz, Dra. Marta Torresan, Dra. Mª Ángeles Gantes, Dra. Inmaculada Ros, Dra. Ana Paula Cacheda, Dra. Mónica Ibáñez Barceló, Dr. Antonio Juan Mas, Dra. Castro Oreiro, Dra. María José Poveda Elics, Dra. Sagrario Bustabad, Dra. María González, Dra. Lorena Expósito, Dr. José Luis Tandaipan Jaime, Dr. Manuel Jose Moreno Ramos, Dra. Paloma Valentina Castillo Dayer, Dr. Luis Francisco Linares Ferrando, Dra. Pilar del Río, Dra. Concepción Delgado Beltrán, Dra. Cilia Amparo Peralta Ginés, Dr. Agustí Sellas, Dra. Nuria Montalà, Dr. Jaime Calvo, Dra. Belén Álvarez Rodríguez, Dr. Fernando Rodriguez Martinez, Dr. Juan Moreno Morales, Dr. Juan Carlos Torre Alonso, Dr. Fernando Jirout Calilla.