Rosai–Dorfman disease (RDD) is uncommon in daily practice, but needs to be ruled out in rheumatologic conditions to elucidate a wide differential diagnosis. Beside its typical presentation, soft tissue masses can be easily seen in our Rheumatology clinics. Ultrasonography widely extended in our specialty, could also play a role in the diagnosis, to end up with the histological confirmation of the disease.
La enfermedad Rosai–Dorfman (ERD) es infrecuente en nuestra práctica diaria, aunque se debe considerar en el diagnóstico diferencial de diferentes procesos en reumatología. La presentación en forma de masa de partes blandas incluye el estudio radiológico y la ecografía de partes blandas muy extendida en nuestra especialidad y, finalmente, la confirmación histológica.
RDD is a benign proliferation of histiocytes, also known as Sinus histiocytosis with massive lymphadenopathy, commonly present in young adults. Is rare as isolated cutaneous presentation and appears with hyperpigmented or erythematous papules, nodules or plaques.1 Affects lymph nodes as well as extra-nodal areas as a systemic disease with fever, leukocytosis, elevated erythrocyte sedimentation rate (ESR), and polyclonal hypergammaglobulinemia.
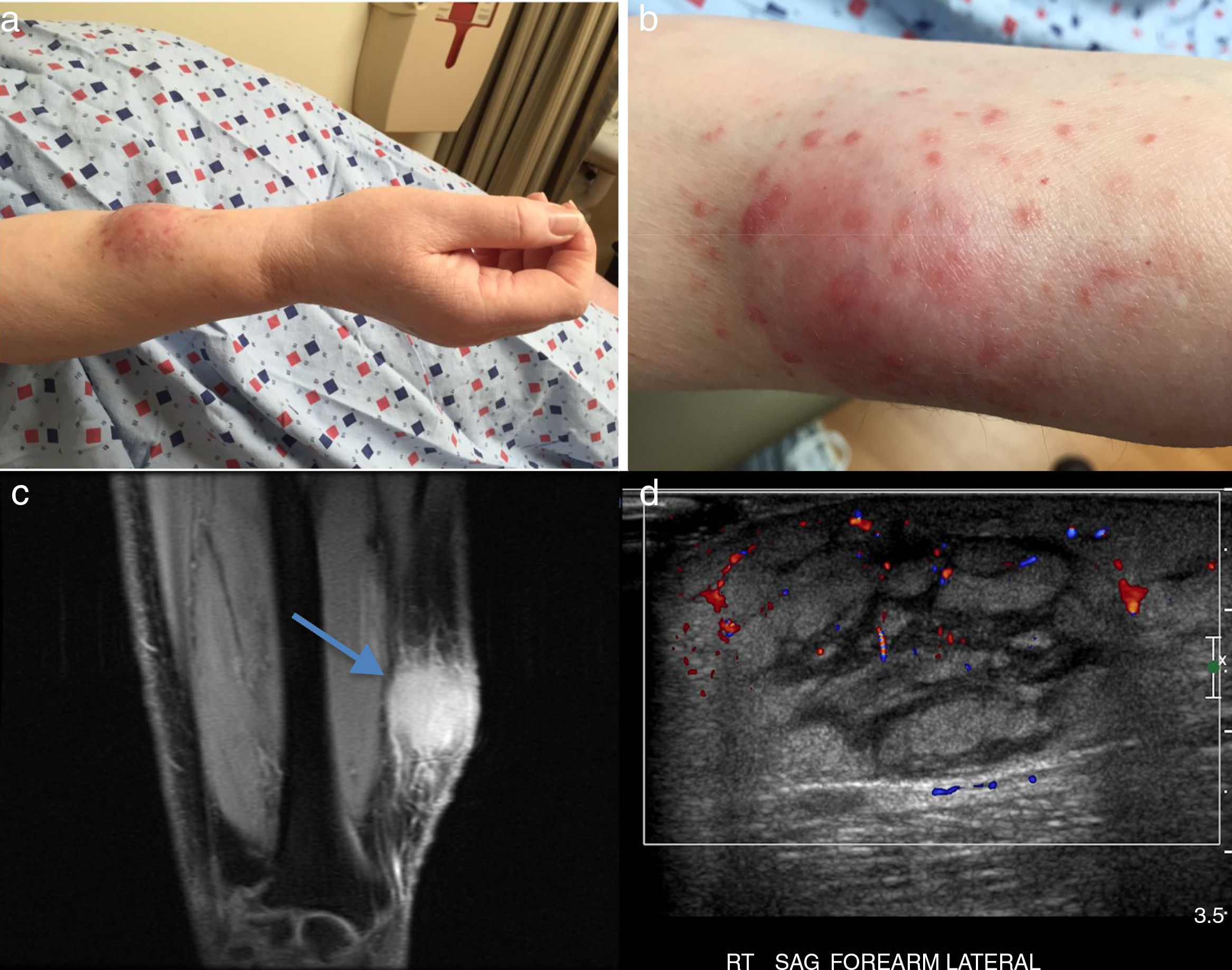
Case reportA 62-year-old Hispanic female developed a small lesion on her right forearm on December 2014. The lesion increased in size significantly over a three weeks period becoming a large soft tissue mass and she developed multiple red spots at the site of the mass (Fig. 1a and b). In February 2015, an MRI showed a T1 Fat suppression hypointense and T2 hyperintense lesion with a discrete mass and enhancement in post gadolinium coronal views with edema, ulceration along the superficial fascia. A gray scale and power Doppler (PD) ultrasound (US) a month later, showed increased soft tissues thickness with enlargement of the fat pad and septa, with slight PD signal in the periphery (Fig. 1c and d). A high definition US guided-biopsy was performed showing a nonspecific polymorphic inflammation with plentiful macrophages. Gram, Gomori Methenamine Silver (GMS) and Acid-Fast Bacilli (AFB) stains, were all negative. Other immunostains and immunophenotyping (haematoxlin eosin) studies described to date were non-diagnostic (Fig. 2a–e).2
(a) Enlarged soft tissue tumor in the right forearm with small papules, (b) detailed view of the same lesion (c) (a) MRI T1 FS coronal view, showing enhancing lesion without a discrete mass in the right forearm with edema, ulceration along the superficial fascia as well as along the subcutaneous fat with skin thickening (blue arrow). (d) Ultrasonography of the dorsal forearm showing increased soft tissues with thickening of the fat pad and enlarged septa, with slight power Doppler signal in peripheral areas.
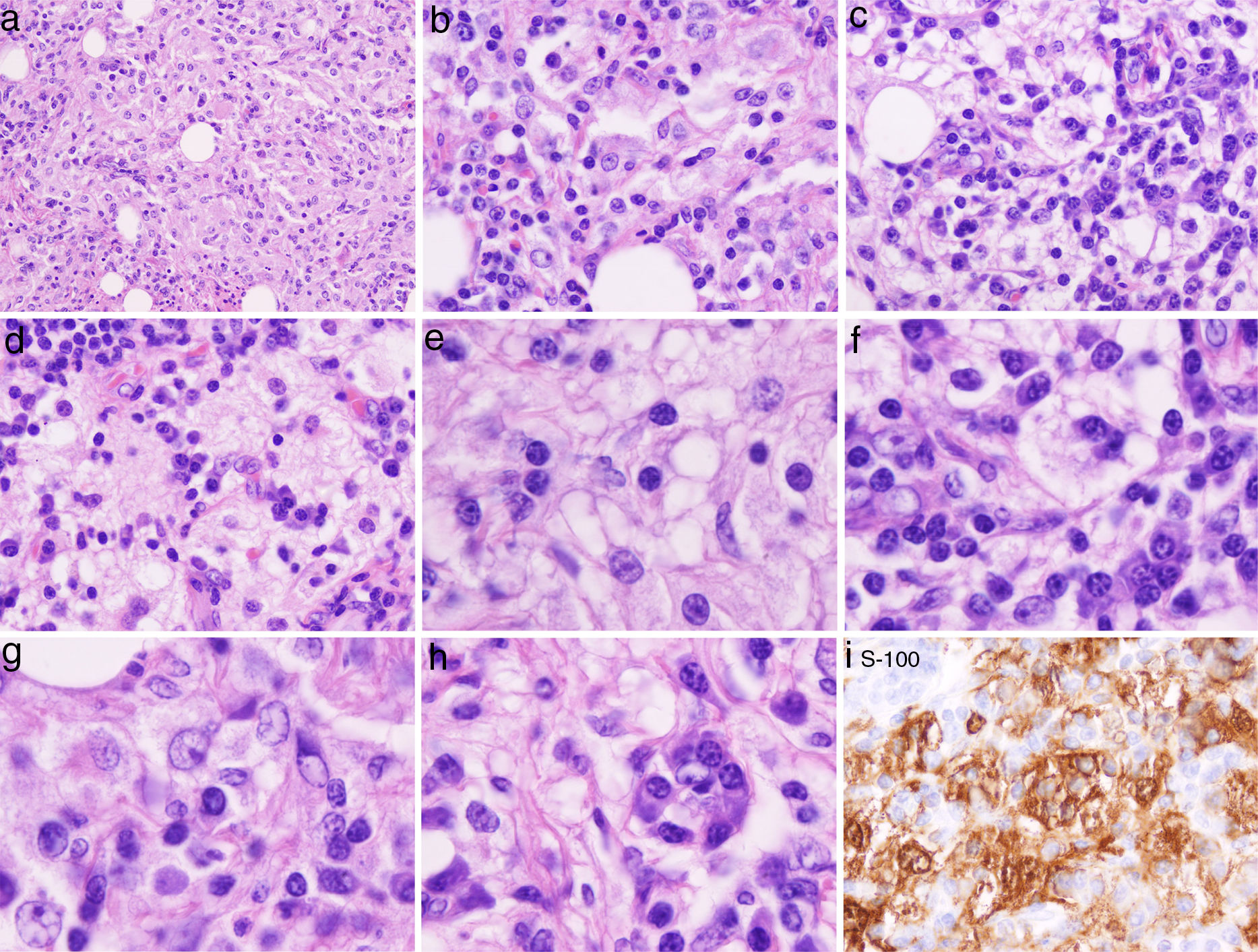
(a, b, c, d) H&E stain low power: infiltration of the dermis or subcutaneous tissue with associated sclerosis: histiocytes, lymphocytes and fibrosis is in the background. Admixed with these large histiocytes there are lymphocytes, plasma cells, neutrophils and eosinophils. (e, f, g, h) High power of the H&E sections: histiocytes containing lymphocytes and plasma cells within a slightly eosinophilic cytoplasm, foam cell with bubbly palely eosinophilic cytoplasm abundantly granular with open vesicular nuclei with prominent nucleoli. (i) S100 stain highlights the macrophages and histiocytes seen on the H&E stain. Lymphocytes, and stained histiocyte containing other cells (emperipolesis) known also as the Rosai–Dorfman (RD) cell.
Work up revealed only ANA 1:640 with no evidence of an underlying inflammatory process or autoimmune disease, such as antiphospholipid syndrome and IgG4 associated sclerosis. A surgical excision of right forearm 7×5cm tumor showed multinodular lesion with thick histiocyte and fibroblasts rich septa. Each nodule contained extended sheets of large, foamy and epithelioid histiocytes, many containing lymphocytes and some plasma cells in the cytoplasm, a phenomenon known as emperipolesis. Foam cell with palely eosinophilic bubbly cytoplasm abundantly granular with open vesicular nuclei with prominent nucleoli were also observed (Fig. 2e–i). Based on the presence of the Protein S-100 and other histologic findings the diagnosis of Rosai–Dorfman disease (RDD) was made.
DiscussionRDD is a benign proliferation of histiocytes, which affects lymph nodes as well as extra-nodal areas.1,2 Sometimes it presents as systemic disease with massive cervical lymphadenopathy with fever, leukocytosis, elevated erythrocyte sedimentation rate (ESR), and polyclonal hypergammaglobulinemia, affecting any age group, but it has a predilection for young adults (mean age 20.6 years); cutaneous form is more common in Asians.
The hallmark of RDD is emperipolesis, defined as the presence of an intact cell within the cytoplasm of another cell. Emperipolesis is unlike phagocytosis, in which the engulfed cells are killed by the lysosomal enzymes of the macrophage. Presence of plasma cells is frequently dense in cutaneous RDD. Thus, some experts consider that a tight relationship exists between RDD disease and IgG4-related sclerosing diseases. The differential diagnosis includes a wide range of heterogeneous diseases such as inflammatory pseudotumor, malignant histiocytosis and lymphoma, hemophagocytic syndrome associated with T-cell lymphoma and/or viral infection, Langerhans cell histiocytosis, Reticulohistiocytoma cutis, Eruptive xanthoma, generalized eruptive histiocytoma, Juvenile xanthogranuloma, inflammatory malignant fibrous histiocytoma, Lepromatous leprosy, Hodgkin's lymphoma. The most distinguishing feature for RD compared to those entities is the presence of Emperipolesis and of protein S-100.3,4
The outcome is described in three different stages: growing (half to one year), full-blown (12 and 24 months), when the polymorphous infiltrating cells and RD cells gradually decrease. Some fibrosis and foam cells may appear in the late regressing phase where lesions are nodule or plaques imparting a yellowish hue, and scar-like tissue.5–7 A recent review has proposed a new classification of histiocytoses according to histology, phenotype, molecular alterations, clinics and imaging characteristics, including: (a) Langerhans-related, (b) cutaneous and mucocutaneous, (c) malignant histiocytoses, (d) Rosai–Dorfman disease, (e) hemophagocytic lymphohistiocytosis and macrophage activation syndrome.8 Follow up visit at month 3 after surgical scission and oral steroids revealed full vanishing of the tumor and complete recovery.
ConclusionRDD is not well recognized and sometimes misdiagnosed as inflammatory pseudotumor. Histological diagnosis is critically necessary, nonetheless our case supports the potential role for ultrasonography in Rheumatology outpatient clinics. Empiric treatment including systemic or intralesional steroids, chemotherapy, radiation therapy and thalidomide have been tried.3,9 Interestingly RDD, can resolve spontaneously, over a long period of time, two years or longer, especially this cutaneous form.10
Conflict of interestThe authors declare that they have no conflict of interest.