Systemic sclerosis (SSc) is a progressive autoimmune connective tissue disease. Platelets to lymphocytes (PLR) and platelets to haemoglobin ratios (PHR) are emerging biomarkers used in the assessment of activity and severity of various autoimmune diseases. This study was designed to clarify the association of PLR and PHR with SSc disease activity and its different manifestations.
MethodA cross-sectional study involved sixty SSc patients. Demographic, clinical data and investigations were done.
ResultsPLR and PHR were correlated positively with ESR (r=0.351, p=0.003*), (r=0.620, p=0.000**), CRP (r=0.417, p=0.001*), (r=0.305, p=0.018**) and SSc activity index (r=0.292, p=0.024*), (r=0.359, p=0.005*). PLR and PHR were highly significantly related to digital ulcerations, musckeloskeletal, and pulmonary manifestations. Also, they had a significant relation to ground glass abnormalities on HRCT, mild restriction in pulmonary function tests and anti-scleroderma-70 antibodies. The cutoff value for PLR was 107.8 with high sensitivity 97.9% and specifity 92.3%, area under the curve (AUC=0.723, P 0.015) on receiver operating characteristic curve (ROC). PHR AUC (0.799, P .001), cut value was 23.5 at 95.7% sensitivity and 84.6% specifity.
ConclusionPLR and PHR were significantly related to digital ulcerations, musculoskeletal, and pulmonary manifestations and can be considered as predictive biomarkers for the assessment of SSc disease activity and severity.
La esclerosis sistémica (ES) es una enfermedad autoinmune progresiva del tejido conectivo. Las proporciones de plaquetas a linfocitos (PLR) y de plaquetas a hemoglobina (PHR) son biomarcadores emergentes que se utilizan en la evaluación de la actividad y la gravedad de diversas enfermedades autoinmunes. Este estudio fue diseñado para aclarar la asociación de PLR y PHR con la actividad de la enfermedad de ES y sus diferentes manifestaciones.
MétodoEstudio transversal en el que participaron 60 pacientes con ES. Se realizaron investigaciones y datos demográficos, clínicos.
ResultadosPLR y PHR se correlacionaron positivamente con la VSG (r=0,351; p=0,003 *), (r=0,620, p=0,000 **), CRP (r=0,417; p=0,001 *), (r=0,305; p=0,018 **) e índice de actividad de ES (r=0,292; p=0,024 *), (r=0,359; p=0,005 *). PLR y PHR se relacionaron de manera muy significativa con ulceraciones digitales, manifestaciones musculoesqueléticas y pulmonares. Además, tenían una relación significativa con las anomalías en vidrio esmerilado en la TCAR, la restricción leve en las pruebas de función pulmonar y los anticuerpos antiesclerodermia-70. El valor de corte para PLR fue 107,8 con alta sensibilidad (97,9%) y especificidad (92,3%), área bajo la curva (AUC=0,723; p=0,015) en la curva característica de funcionamiento del receptor (ROC). PHR AUC (0,799; p=0,001), el valor de corte fue 23,5 con una sensibilidad del 95,7% y una especificidad del 84,6%.
ConclusiónPLR y PHR pueden considerarse biomarcadores predictivos para la evaluación de la actividad y la gravedad de la enfermedad de ES.
Systemic sclerosis (SSc) is a rare rheumatic disease caused by innate and adaptive immune system disturbance, vasculopathy with endothelial dysfunction, and collagen deposition in the extracellular matrix resulting in fibrosis of the skin and internal organs.1
Platelets (PLT) are considered to be one of the immune response regulators implicated in the development of SSc, Platelets activation contributes to endothelial dysfunction resulting in reactive oxygen species release with subsequent stimulation of inflammation and collagen deposition leading to fibrosis.2 Activated platelets release mediators that stimulate proliferation of fibroblast and production of collagen as transforming growth factor-beta, platelet-derived growth factor, and other mediators which promote the peripheral microvascular injury progression and poor healing in SSc as fibronectin and thrombospondin-1.3 They are the primary source of serotonin, which boosts the synthesis of collagen, activation T-lymphocytes, and the generation of inflammatory cytokines like tumor necrosis factor-a and interleukin-6.4 Platelets are also a source of the high-mobility group box 1 protein, which causes the damage of endothelial cells and fibrogenesis.5 Platelets to lymphocytes ratio (PLR) and platelets to haemoglobin ratio (PHR) have emerged as new biomarkers of inflammation, which were previously measured using acute phase reactants such as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR).6 They have been investigated, in many autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus, behest's disease and systemic vasculitis and in other diseases as malignancies and cardiovascular diseases to predict the outcome of the inflammatory process and assess the disease activity and severity.7,8 As a result, we hypothesized that PLR and PHR may be used as predictive SSc biomarkers. However, Only a few number of researches have explored the clinical significance of these biomarkers in SSc patients,9 We aimed to investigate if there is any association between these ratios and various manifestations and disease activity in SSc.
Patients and methodsStudy designThis study is a cross-sectional study that included sixty SSc patients. Patients were diagnosed according to ACR/EULAR criteria of SSc.10 Exclusion criteria involved patients diagnosed with other autoimmune disorders or high inflammatory makers due to infection or malignancy. The study was authorized by the local Scientific Research Ethics Committee. All patients were informed about the study by a written consent.
Assessment- •
A full history was taken including (ages, sex, and disease duration) and clinical examination including general, skin tightness assessment by modified Rodnan's score (mRSS), vascular (Raynaud's, pitting scars, digital ulcers), pulmonary, musculoskeletal, gastrointestinal and cardiac assessment.
- •
Investigations involved ANA titer and pattern, Anti scleroderma 70 antibodies, pulmonary function tests (PFTs), High-Resolution Computer Tomography (HRCT) on the chest, and cardiac Echo.
- •
Disease activity assessment by the European Scleroderma Trials and Research Group (EUSTAR) Activity Index (EScSG-AI).11 It is a 10-point score including worsening of skin tightness over the past month, elevated mRSS, tendon friction rub, digital ulcers, high CRP and diffusion lung capacity for carbon monoxide (DLCO) less than 70% of predicted.
Suitable tubes were used. Serum or Plasma with Li-heparin and K2-EDTA plasma are acceptable specimens with centrifugation of samples containing precipitates before performing the assay. Automated cell counter Sysmex XN2000 (Sysmex diagnostic, Japan) was used for CBC differential cell count. NLR and PLR were recorded. To estimate PLR, the platelet count was divided by the lymphocyte cell count. To estimate the PHR, the ratio of platelet count to hemoglobin count was obtained. No specific reference range has been established. CRP detection by an immune turbidimetric assay using an auto-analyzer (Roche Diagnostic, Mannheim, Germany). Vision B (ultra vision diagnostic, China) was used to measure ESR).
Statistical analysisStatistical Package for Social Science (SPSS) (Version 20.0. Armonk, NY: IBM Corp.) was used for coding data and analysis. Qualitative variables were presented as the number and percentage. Quantitative data were displayed (if not normally distributed data) by the mean±standard deviation (SD), median, and range. The Chi-square test (χ2) and the Fisher exact test were used to assess the relationship between various qualitative variables. The Spearman's correlations were calculated to assess the relationship between PLR and PHR with various study variables. The receiving Operating Characteristic (ROC) curve was used to determine the sensitivity and specificity of PHR and PLR, a. The results were considered statistically significant and highly statistically significant when the significant probability (p-value) was <0.05 and <0.001 respectively.
ResultsDemographic, clinical data, investigations of patients were demonstrated in Table 1. The mean age of patients was 40.8±9.8years, and the mean period of the disease was 5.4±4.9. The majority of patients (85 percent) were females. Raynaud's phenomenon present in 91.7% of patients, gastrointestinal tract (GIT), musculoskeletal and pulmonary manifestations present in 70%, 60%, and 56.7% of patients respectively, the mean modified Rodnan's score was 18.9±7.5. ANA and Anti-Scleroderma 70 antibodies were detected in 91.6% and 41.6% of patients respectively. Also, early ground-glass abnormalities on HRCT and mild restriction of PFTs were found in 42.8% and Echo abnormalities in 32.9% of patients, Table 1.
Systemic sclerosis patients demographic and clinical features (n=60).
| Characteristics | Value |
|---|---|
| Age (years): Mean±SD | 40.8±9.8 |
| (Range) | 24–60 |
| Female sex: No (%) | 51 (85%) |
| Disease duration (years): Mean±SD | 5.4±4.9 |
| (Range) | 2–20 |
| Diffuse SSC: No (%) | 48 (80) |
| Mucocutanous manifestations | |
| Raynaud's | 55 (91.7) |
| Puffines | 55 (91.7) |
| Ulceration | 13 (21.7) |
| Gangrene | 10 (16.7) |
| Pitting scars | 38 (63.3) |
| Telengectsia | 5 (8.3) |
| Calcinosis | 2 (3.3) |
| Modified Rodnan's score | |
| Mean±SD | 18.9±7.5 |
| (Range) | (6–34) |
| GIT manifestations | 42 (70) |
| Chest manifestions | 34(56.7) |
| Musckeloskeletal | 36 (60) |
| WBCS×103/μL | |
| Mean±SD | 8.51±3.2 |
| (Range) | 5–17 |
| Median | 7.7 |
| Haemoglobin g/dL | |
| Mean±SD | 11.10±1 |
| (Range) | 9–14 |
| Median | 11 |
| Platelets×103/μL | |
| Mean±SD | 360.4±73 |
| Range | 200–555 |
| Median | 383 |
| ANA positive | 55 (91.6) |
| Positive Anti-Scleroderma 70 | |
| No (%) | 25 (41.6) |
| Mean±SD | 87.9±86.75 |
| (Range) | (0.1–242.4) |
| EScSG-AI | |
| Mean±SD | 3.3±1.6 |
| (Range) | 0.5–7.5 |
| PFT: No (%) | |
| Normal | 26 (43.3) |
| Mild restriction | 29 (48.4) |
| Moderate restriction | 5 (8.3) |
| HRCT: No (%) | |
| Normal | 30 (50.0) |
| Early ground | 26 (43.3) |
| Honey coomb | 4 (6.7) |
GIT: Gastrointestinal Tract, EScSG-AI: European Scleroderma Trials and Research Group (EUSTAR) Activity Index, HRCT: High Resolution Computed Tomography, PFT: Pulmonary Function Test.
Regarding the inflammatory markers among SSc patients, the mean ESR was 32.2±9.5, the mean CRP mean was 7.0±2.2. PLR means was 245.07±142.5 ranged from 100 to 975, its median was 209.7. PHR mean was 32.96±8.82, its range (16.7–61.1) and its median was 31.7, Table 2.
Correlations of PLR, PHR with various disease parameters are shown inTable 3. PLR, PHR were correlated positively with ESR (r=0.351, p=0.003*) (r=0.620, p=0.000 **), CRP (r=0.417, p=0.001*), (r=0.305, p=0.018 **), EScSG-AI activity score (r=0.292, p=0.024*), (r=0.359, p=0.005*). PHR was correlated negatively with HB (r=0.640, p=0.000**) and correlated positively with PLT (r=0.929, p=0.000**).
Correlation between PLR, PHR and patients charachteristics among SSC patients.
| Character | PLR | PHR | ||
|---|---|---|---|---|
| r | p | r | p | |
| Age | −.074 | 0.57 | −.074 | 0.57 |
| Disease duration | −.061 | −0.645 | −.184 | −0.16 |
| ESR | .351 | 0.003* | .620 | 0.000** |
| CRP | .417 | 0.001* | .305 | *.018 |
| WBCS | −.025 | −0.85 | .070 | 0.59 |
| HB | −.136 | 0.3 | −.640 | 0.000** |
| PLT | .196 | 0.13 | .929 | 0.000** |
| Modified Rodnans score | −.006 | −0.966 | −.016 | −.905 |
| EScSG-AI | .292 | 0.024* | .359** | 0.005* |
In bold, significant correlation.
The relation between PLR, PHR, and different SSc disease manifestations revealed that PLR, PHR were highly significantly related to skin ulcerations, musculoskeletal, and pulmonary manifestations (p<0.05). Also, they significantly related with ground glass abnormalities on HRCT and mild restriction in pulmonary function tests and antiscleroderma 70 (p<0.05), Table 4.
Relation between PLR, PHR and visceral involvements among SSC patients (n=60).
| Visceral involvements | PLR | PHR | ||||
|---|---|---|---|---|---|---|
| No (%) | Test | P | No (%) | Test | P | |
| Raynaud's (n=55) | 30 | a13.47 | 0.000** | 28 | a2.25 | 0.13 |
| Ulceration (n=13) | 10 | b5.02 | 0.025* | 11 | b7.83 | 0.005* |
| Musckeloskeletal (n=36) | 20 | a1.11 | 0.29 | 21 | a1.60 | 0.21 |
| GIT (n=42) | 20 | a0.32 | 0.57 | 20 | a0.918 | 0.34 |
| Pulmonary (n=34) | 12 | a6.79 | 0.009* | 26 | a17.21 | 0.000** |
| Diffuse type (n=48) | 24 | a2.86 | 0.09 | 25 | a3.46 | 0.63 |
| Antiscleroderma 70 (=25) | 18 | a8.3 | 0.004* | 21 | a17.49 | 0.000** |
| HRCT | ||||||
| Normal (n=30) | 9 | b9.84 | 0.00** | 9 | a11.7 | 0.003** |
| Early ground (n=26) | 18 | 19 | ||||
| Honey coomb (n=4) | 3 | 3 | ||||
| PFT | ||||||
| Normal (n=26) | 9 | b8.25 | 0.016* | 7 | b11.51 | 0.000** |
| Mild restriction (n=29) | 18 | 21 | ||||
| Moderate restriction (n=5) | 3 | 3 | ||||
| Abnormal echo (n=16) | 10 | b0.31 | 0.57 | 9 | b0.34 | 0.58 |
The effect of drugs on PLR and PHR among SSC patients showed in Table 5, we found no significant difference when comparing PLR and PHR means in patients who received each drug (corticosteroids, azathioprine, cyclophosphamide and mycophenolate mofetil) and those not receiving each drug. Therefore, we were not able to completely rule out the possibility that differences in blood cells-derived indexes may, at least in part, be attributable to concomitant conditions.
The effect of drugs on PLR and PHR among SSC patients.
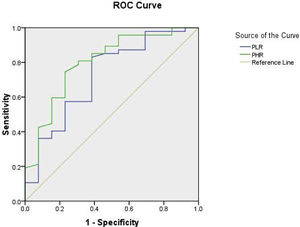
As regard the cut-off values for PLR, receiver operating characteristic curve (ROC) interpretation revealed a high sensitivity 97.9% and specifity 92.3% at optimum cut off value 107.8, area under the curve (AUC=0.723), (p=0.015), which was highly significant with 95 percent confidence interval. PHR AUC (0.799, p .001) cut-off value: 23.5 at 95.7% sensitivity and 84.6% specifity as shown in Fig. 1.
DiscussionPLR and PHR have been postulated as an inflammatory biomarkers in a variety of autoimmune illnesses, where aberrant lymphocyte signalling leads to cell-mediated and autoantibodies productions autoimmunity and active platelets secrete inflammatory mediators, amplify cytokine release12 and provide B cells costimulation by expressing CD40L with subsequent antibodies production.13
In the current study, the mean patient's age was 40.8±9.8years. Pulmonary manifestations present in 56.7% of patients, the mean modified Rodman's score was 18.9±7.5, digital ulcers present in 21.7%. In line with Sakr et al.,14 who stated that the SSc patient's mean age was 42.8±12.6. Also, Kim et al.15 revealed that (30.7%) of SSc patients had digital ulcers and (47.4%) developed ILD.
Regarding the inflammatory markers among SSc patients in the present study, PLR mean was high. This thrombocytosis is a result of the release of the inflammatory cytokines as TNF-a and IL-8.16 In agreement with Sakr et al.14 who found that PLR was high in SSc, Also, ankylosing spondylitis16 and takayasu vasculitis17 have both been linked to PLR elevation.
In the present study, PLR was correlated positively with ESR, CRP, EScSG-AI activity scores. The relation between PLR and different SSc disease manifestations revealed that PLR was highly significantly related to digital ulcerations, musculoskeletal, and pulmonary manifestations. Also, they significantly related with ground glass abnormalities on HRCT and mild restriction in PFTs and antiscleroderma 70 (p<0.05).
Similarly, Sakr et al.14 and Peng et al.16 reported that PLR levels were found to be considerably greater in patients with myositis and proximal muscle weakness. In agreement with Kim et al.15 who stated that PLR was associated with the digital ulcers and interstitial lung disease development in SSc patients, Anti-Scl70-positive patients. However, the PLR showed no significant association with the RSS score, pulmonary hypertension, and gastrointestinal manifestations
Although no significant relation was found between cardiac affection by Echo and PLR in the current research, a higher number of patients with cardiac diseases had high PLR. In contrast, another study stated that the platelet count was significantly lower only in CTD-related PAH, being sensitive in excluding PAH this finding might be explained by the local consumption of platelets due to endothelial damage.2
PHR was high in SSc patients and was negatively correlated with haemoglobin and positively correlated with PLT, ESR, CRP, EScSG-AI activity score, Salvagno et al.17 stated that that the lower the hemoglobin level, the higher the general severity score of SSc. Up to our knowledge, no previous studies assessed the significance of PHR in SSc.
As regard the cut off values for PLR, ROC interpretation revealed a high sensitivity 97.9% and specificity 92.3% at optimum cut off value 107.8, (AUC=0.723), (p=0.015), which was highly significant. In disagreement with a study done in Behcet's disease18 and reported a higher PLR cutoff, 144.05 with lower sensitivity (62.5%) and specificity (57.1%), area under the curve (AUC=0.625) and p=0.350 which was insignificant, and another study done on systemic lupus erythematosus19 which revealed the best PLR cut-off value was 132.9.
Immunosuppressive drugs may have a potential effect on PLR and PHR either by producing bone marrow suppression or by better controlling the inflammatory process. However, we found no significant difference when comparing PLR and PHR means in patients who received immunosuppressive drug. Therefore, we were not able to completely rule out the possibility of concomitant use of multiple drugs or previous use of different drugs. This point was a limitation in previous studies.20
LimitationsThe current study's limitations include a small sample size, the need for results to be confirmed on a larger cohort of SSc patients, and the possibility of a more longitudinal follow-up study to further analyze the relationship between disease severity and these ratios also to accurately assess the potential impact of specific immunosuppressive drugs on these indices.
ConclusionPLR and PHR were significantly related to digital ulcerations, musculoskeletal, and pulmonary manifestations and can be considered as a predictive biomarker for the assessment of disease activity in SSc.
Ethical standardsOfficial permission was obtained from Institutional Review Board (IRB) at the Faculty of Medicine, Zagazig University Hospitals, and from the Rheumatology& Rehabilitation Department. The study has been carried out by The Code of Ethics of the World Medical Association (Declaration of Helsinki 1964) for studies involving humans in research. Written informed consent was obtained from the participants.
Significance of Platelets to Lymphocytes and Platelets to Haemoglobin Ratios in Systemic Sclerosis Patients.
Funding informationThe authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interestThe authors declare that they have no conflict of interest.