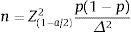To describe the status of using biological Disease Modifying Anti Rheumatic Drugs (bDMARDs) to treat rheumatoid arthritis (RA) and related factors. In addition, the study determined the impact of COVID-19 on the usage of bDMARDs.
MethodsThis is a cross-sectional study and included 219 RA patients over 18 years old. The Kaplan–Meier method and the log-rank test (p<0.05) were used to estimate the retention time and compare between different times. Cox regression analysis was used to determine the factors affecting the retention time of biological drugs (p<0.05).
ResultsOut of 1967 courses of treatment, there were 149 (7.6%) drug discontinuations, 760 (38.6%) doses extensions and 64 (3.3%) drug switch. Moderate disease level and choosing tumor necrosis factor (TNF) inhibitors initially were associated with retention time of COVID-19. Drug discontinuations and dose extensions increased after COVID-19 emergence. The retention time during COVID-19 was significantly different from that of pre-COVID-19. Gender, type of first-used bDMARD, conventional synthetic DMARDs (csDMARDs) and corticoid usage status, disease activity levels were associated with retention time.
ConclusionThe presence of COVID-19 has a significant effect on usage status of the biologic drug. Further longitudinal studies are needed to clarify the relationship between COVID-19 and drug usage as well as related factors.
Describir el estado del uso de fármacos antirreumáticos modificadores de la enfermedad biológica (bDMARD) para tratar la artritis reumatoide (AR) y los factores relacionados. Además, el estudio determinó el impacto de COVID-19 en el uso de bDMARD.
MétodosEste es un estudio transversal que incluyó a 219 pacientes con AR mayores de 18 años. El método Kaplan-Meier y la prueba Log-rank (p<0,05) se usaron para estimar el tiempo de retención y compararlo entre diferentes tiempos. El análisis de regresión de Cox se utilizó para determinar los factores que afectan el tiempo de retención de los medicamentos biológicos (p<0,05).
ResultadosDe 1.967 cursos de tratamiento, hubo 149 (7,6%) interrupciones del fármaco, 760 (38,6%) extensiones de dosis y 64 (3,3%) cambios de fármaco. Nivel de enfermedad moderado y elección del factor de necrosis tumoral (TNF) inhibidores inicialmente se asociaron con el tiempo de retención de COVID-19. Las discontinuaciones de los medicamentos y las extensiones de las dosis aumentaron después de la aparición de COVID-19. El tiempo de retención durante COVID-19 fue significativamente diferente del pre-COVID-19. Género, tipo de bDMARD de primer uso, convencional DMARD sintéticos (csDMARDs) y el estado de uso de corticoides, los niveles de actividad de la enfermedad se asociaron con el tiempo de retención.
ConclusiónLa presencia de COVID-19 tiene un efecto significativo en el estado de uso del medicamento biológico. Se necesitan más estudios longitudinales para aclarar la relación entre COVID-19 y el uso de fármacos, así como los factores relacionados.
Rheumatoid arthritis (RA) is an autoimmune type of arthritis that is caused by both genetic and extrinsic environmental factors.1 About 1% of the population is suffering from the consequences of this disease. People with RA are at high risk of disability, loss of work capacity, and death.2 Therefore, biological Disease Modifying Anti Rheumatic Drugs (bDMARDs) have been developed as an effective method to improve treatment outcomes and prognosis of the disease.2,3 Currently approved bDMARDs have four modes of action: (1) tumor necrosis factor-α (TNF-α) inhibitors; (2) interleukin-6 (IL-6) inhibitors and interleukin-6 receptor (IL-6R) inhibitors; (3) inhibitors of co-stimulatory molecules; and (4) B-cell depletion antibodies. Some studies showed that the IL-6 inhibitors group is often chosen first when starting treatment.4–6 In addition, the rate of discontinuations, drug switching and retention time were evaluated as well. Multiple factors including sex, obesity, prior glucocorticoid use, and starting TNF-α inhibitors were strongly associated with retention time.5,6 Several other studies have compared the effectiveness of bDMARDs with other DMARDs. bDMARDs have been shown to be effective in reducing mortality and stroke compared with controls not receiving bDMARDs, while conventional synthetic DMARDs (csDMARDs) were associated with increased risk of mortality compared with controls.7
Vietnam was one of countries having low biologic and targeted synthetic DMARDs (b/tsDMARDs) prescription. Compared to other countries in APLAR RA SIG group (including China, Singapore, Thailand, Japan, Bangladesh, Indonesia, Malaysia, Kuwait and Nepal, and India), patients from Vietnam had low usage of b/tsDMARDs while having higher rates of GC usage which was co-prescribed with csDMARDs.8 Unfortunately, we could not find any research about factors on the usage status of bDMARDs for RA in Vietnam.
During the COVID-19 pandemic, the authorities began postponing and reducing outpatient visits and non-emergency surgery to relieve the burden on healthcare systems and to lower the infection risk.9 For example, visits in outpatients’ emergency department from January to August in 2020 was lower than in 2019 in Utah by 8.1%, and the largest reduce was in April 2020 with 30.4%.10 In this pandemic, it is important to care for COVID-19 as well as non-COVID-19 patients,11 including maintaining medication especially for patients suffering from autoimmune diseases like RA. Although it has to be balanced with the concerns of preventing SARS-CoV-2 spread and protecting patients.12 In the United States, 14.9% of patients discontinued DMARDs and more frequent stopping was associated with bDMARDs/Janus kinase inhibitor and greater concern about COVID-19.13 In term of using bDMARDs in RA patients, researchers had shown a high rate of drug discontinuation during COVID-19 pandemic. It was reported that 13.8% of RA patients in Treasure registry of Turkey discontinued their bDMARDs.14 Furthermore, comparing the rate of bDMARDs weekly use during 2019–2020 in Lazio, Italy, a decrease of 25.5% in RA patients was reported during lockown.15 In Denmark, the percentage of patients reported to have changed bDMARDs dosage was 6% in March 2020 and about half of patients changed their dosages due to fear of COVID-19.16
In general, bDMARDs are very important in the treatment of RA and have only been applied in Vietnam for a short time, thus there were few studies about this topic. Moreover, the COVID-19 pandemic has a great impact on clinical visit of RA outpatients. Thus, we conducted this study on RA patients in Vietnam with two objectives: firstly, to evaluate the patient's ability to maintain bDMARDs medication during the COVID-19 pandemic, and secondly to find out related factors affecting their abilities. In addition, research on the effect of this pandemic on the usage of bDAMRDs is essential to assess the consequences on patients’ health status.
Subjects and methodsStudy populationThe study population included 219 RA patients aged 18 years old or older who had used biological drugs at the Department of Rheumatology in Bach Mai Hospital from January 2017 to December 2020 and did not have other autoimmune diseases. Such patients had sufficient medical records from the first bDMARD treatment, consented to participate in the study and answered all questions in the study medical records. The research was approved by the Ethics Committee in Biomedical Research of Bach Mai Hospital according to Decision numbered: 2051/BVBM-HĐĐĐ.
Method- -
Study design: a cross-sectional study with retrospective and prospective data collection.
- -
Sample size: we use the formula belowed to estimate a proportion.
In there:
n: minimum sample size needed.
α: statistical significance level. In this study, α=0.05 (95% confidence level).
Z(1−α/2)=1.96 corresponding to the level of statistical significance α=0.05.
Δ: random error, we choose Δ=0.05.
p1: percentage of patients who started treatment with a biologic drug of the TNFi group in a previous study (95.4%),17 from which n=68 was calculated. Thus, 68 patients were the minimum sample size. In this study, we recruited a total of 219 patients.
- -
Place of study: the Department of Rheumatology in Bach Mai Hospital.
- -
Sample collection: we use purposeful sampling: RA patients who satisfy the selection criteria treated with biological drugs at the Department of Rheumatology in Bach Mai Hospital from January 1, 2017, to the end of December 31, 2020 were recruited.
- -
Some definitions:
- •
Dose tapering: the interval in which the previous course of treatment is longer than recommended.18
- •
Drug discontinuation: the interval in which the previous treatment is three times longer than recommended.18
- •
Drug switch: when a patient switch to a different drug and does not stop taking it.
- •
Overall bDMARDs retention time: time from the first dose of first-used bDMARD to the last dose of last-used bDMARD.
- •
First-used bDMARD retention time: time from the first dose to the last dose of first-used use bDMARD.
- •
The data were entered into Excel and SPSS 20 software was used for analysis. To estimate the retention time and compare between groups, we used the log-rank test (p<0.05) and the Kaplan–Meier method. Cox regression analysis was used to determine the factors affecting the time patients maintained biological drugs (p<0.05).
ResultsCharacteristics of studied subjectsOut of 219 RA patients, 85.4% were females, and the majority (91.8%) of patients were more than 40 years old. The proportions of patients in urban and rural areas were 50.7% and 49.3%, respectively. The percentage of patients having a history of tuberculosis or latent tuberculosis was 13.2%, hepatitis B was 3.7% and cancer was 0.5% (Table 1). The vast majority of patients had moderate to high disease activity level, while only 8.2% was at low level. Most of patients was firstly prescribed with an IL-6 inhibitor (78.5%) and 83.6% of patients were using corticoid.
Demographic characteristics of the study subjects.
| Demographic factors | n (%)(n=219) |
|---|---|
| Sex | |
| Male | 32 (14.6) |
| Female | 187 (85.4) |
| Age | |
| <40 | 18 (8.2) |
| 40–59 | 117 (53.4) |
| ≥60 | 84 (38.4) |
| Area | |
| Urban | 111 (50.7) |
| Rural | 108 (49.3) |
| Past medical history factors | n (%)(n=219) |
|---|---|
| Tuberculosis or latent tuberculosis infection | |
| No | 190 (86.8) |
| Yes | 29 (13.2) |
| Hepatitis B | |
| No | 211 (96.3) |
| Yes | 8 (3.7) |
| Cancer | |
| No | 218 (99.5) |
| Yes | 1 (0.5) |
| Onset time | |
| <6 months | 37 (16.9) |
| ≥6 months | 182 (83.1) |
| Disease activity level | |
| Low | 18 (8.2) |
| Moderate | 101 (46.1) |
| High | 100 (45.7) |
| First-used bDMARD | |
| IL-6 inhibitors | 172 (78.5) |
| Tocilizumab | 172 (78.5) |
| TNF inhibitors | 47 (21.5) |
| Infliximab | 20 (9.1) |
| Adalimumab | 15 (6.8) |
| Golimumab | 12 (5.5) |
| Number of csDMARD at the start of treatment | |
| 0 | 26 (11.9) |
| 1 | 80 (36.5) |
| 2 | 105 (47.9) |
| 3 | 8 (3.7) |
| Corticoid at the start of treatment | |
| No | 36 (16.4) |
| Yes | 183 (83.6) |
| VAS score | |
| Low (10–40mm) | 112 (51.1) |
| Moderate (50–70mm) | 106 (48.4) |
| High (80–100mm) | 1 (0.5) |
Among a total of 1967 biologic courses of treatment, there were 149 (7.6%) drug discontinuations, 760 (38.6%) dose tapering, and 64 (3.3%) drug switchs. Using the Kaplan–Meier method to estimate the overall bDMARDs retention time and first-used bDMARD retention time, the data showed 49 weeks (95% CI: 37.83–60.17) and 34 weeks (23.51–44.49) respectively (Fig. 1A–C).
The usage status of biologic drug therapies for rheumatoid arthritis. (A) Study subjects’ usage status of biologic drug based on treatment sessions; (B) probability of overall bDMARDs maintaining over time using Kaplan–Meier method; (C) probability of the first-used bDMARD maintaining over time using Kaplan–Meier method.
The log-rank test showed that there was a statistically significant difference in discontinuation time between the pre- and during COVID-19 groups in both the overall bDMARDs and first-used bDMARD (p=0.047, and 0.039, respectively) (Table 2).
The impact of the COVID-19 pandemic on the retention time of biologic drugs.
Beside the retention time, we also compared the number of other events occurred during bDMARDs treatment between two times. The rates of drug discontinuation and dose tapering before the COVID-19 pandemic were lower than during the pandemic with p<0.001 (Table 3). However, the rates of drug switch before the pandemic and during the pandemic were not statistically different (p>0.05).
Impact of the COVID-19 pandemic on the study subjects’ use of biologic drugs.
| Time | Before the COVID-19 pandemic(n=1388) | During the COVID-19 pandemic(n=579) | p-Value |
|---|---|---|---|
| Drug use status, n (%) | |||
| Normal | 813 (58.6) | 245 (42.3) | <0.001** |
| Discontinuation | 65 (4.7) | 84 (14.5) | |
| Tapering | 510 (36.7) | 250 (43.2) | |
| Drug change status, n (%) | |||
| Normal | 1342 (96.7) | 561 (96.9) | 0.89 |
| Switching | 46 (3.3) | 18 (3.1) | |
**The Chi-square test was used to compare percentage of normal, discontinuation, and tapering, p<0.001.
Of the 119 patients who started using biologic drugs before the COVID-19 pandemic, female patients had a longer retention time than male (HR=0.554, p<0.05). Patients who started treatment with a TNF inhibitor had a shorter overall retention time than those started with an IL-6 inhibitor (HR=1.663, p<0.05). Patients who used 1, 2, and 3 csDMARDs had a longer duration of maintenance on biologic drugs than those treated without csDMARDs (HR=0.302, 0.388, and 0.05; p=0.009, 0.046, and <0.001, respectively). People who used corticoid at the start of treatment had a shorter retention time than those who used non-csDMARDs (HR=4.086, p=0.002). The factors of location, duration of disease, disease activity levels, number of swollen joints, number of tender joints number of csDMARDs used, glucocorticoid use. Visual Analogue Scale (VAS) score did not affect first-used bDMARD retention time (Table 4).
Factors associated with overall and first-used bDMARD retention time as determined by Cox multivariate regression analysis in pre-COVID-19 patients.
| Related factors(p-value<0.05) | Overall bDMARDs retention time (n=119) | First-used bDMARD retention time (n=146) | ||||
|---|---|---|---|---|---|---|
| β | HR (95% CI) | p-Value | β | HR (95% CI) | p-Value | |
| Age at the start of treatment | ||||||
| <40 | 1 | 1 | ||||
| 40–59 | 0.082 | 1.086 (0.521–2.261) | 0.826 | −0.745 | 0.475 (0.236–0.956) | 0.037*** |
| ≥60 | 0.195 | 1.215 (0.608–2.428) | 0.581 | −0.343 | 0.71 (0.358–1.406) | 0.325 |
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | −0.591 | 0.554 (0.323–0.949) | 0.032*** | −0.583 | 0.558 (0.34–0.918) | 0.022*** |
| Area | ||||||
| Rural | 1 | 1 | ||||
| Urban | −0.042 | 0.959 (0.649–1.416) | 0.823 | 0.027 | 1.028 (0.725–1.456) | 0.878 |
| Onset time | ||||||
| <6 months | 1 | 1 | ||||
| ≥6 months | 0.197 | 1.218 (0.732–2.027) | 0.448 | 0.109 | 1.115 (0.701–1.773) | 0.646 |
| Disease activity at the start of treatment | ||||||
| Remission or low | 1 | 1 | ||||
| Moderate | −0.195 | 0.823 (0.363–1.864) | 0.641 | −0.464 | 0.629 (0.305–1.299) | 0.21 |
| High | −0.999 | 0.368 (0.131–1.034) | 0.058 | −0.614 | 0.541 (0.209–1.4) | 0.205 |
| First-used bDMARD | ||||||
| IL-6 inhibitor | 1 | 1 | ||||
| TNFi | 0.508 | 1.663 (1.005–2.749) | 0.048*** | 0.459 | 1.582 (1.05–2.383) | 0.028*** |
| Numbers of csDMARDs at the start of treatment | ||||||
| 0 | 1 | 1 | ||||
| 1 | −1.197 | 0.302 (0.123–0.745) | 0.009*** | −0.024 | 0.977 (0.463–2.058) | 0.95 |
| 2 | −0.948 | 0.388 (0.153–0.982) | 0.046*** | −0.21 | 0.811 (0.358–1.836) | 0.615 |
| 3 | −2.995 | 0.05 (0.009–0.269) | <0.001*** | −0.772 | 0.462 (0.13–1.64) | 0.232 |
| Corticoid at the start of treatment | ||||||
| No | 1 | 1 | ||||
| Yes | 1.408 | 4.086 (1.68–9.94) | 0.002*** | 0.434 | 1.543 (0.754–3.155) | 0.235 |
| Tender joints at the start of treatment | −0.021 | 0.979 (0.93–1.031) | 0.429 | −0.012 | 0.988 (0.94–1.037) | 0.62 |
| Swollen joints at the start of treatment | 0.048 | 1.049 (0.99–1.112) | 0.106 | 0.027 | 1.027 (0.969–1.089) | 0.368 |
| VAS at the start of treatment | 0.01 | 1.01 (0.839–1.217) | 0.914 | 0.093 | 1.097 (0.921–1.308) | 0.3 |
In 146 patients who started using biologic drugs before the COVID-19 pandemic, patients aged 40–59 had a longer retention time of the first-used bDMARD than those under 40 years old (HR=0.475, p<0.05) while no difference was found between the group of ≥60 years old and under 40 years old (HR=0.71, p>0.05). Female patients had a longer retention time than men (HR=0.558, p<0.05). Patients who started treatment with a TNF inhibitor had a shorter retention time than with an IL-6 inhibitor (HR=1.582, p<0.05). Factors including location, duration of disease, disease activity levels, number of csDMARDs, number of swollen joints, number of tender joints, glucocorticoid usage, and VAS score when starting using bDMARDs did not show any association with the retention time of the first-used bDAMRD (Table 4).
In terms of factors related to overall retention time of 23 patients treated since the pandemic, age, number of swollen joints, number of tender joints, and VAS score failed to be associated with retention time (Table 5).
Factors associated with overall and first-used bDMARD retention time as determined by Cox multivariate regression analysis in 23 during COVID-19 patients.
| Related factors(p<0.05) | Overall retention time (n=23) | First bDMARD retention time | ||||
|---|---|---|---|---|---|---|
| β | HR (95% CI) | p-Value | β | HR (95% CI) | p-Value | |
| Age at the start of treatment | −0.018 | 0.982 (0.921–1.048) | 0.586 | −0.018 | 0.982 (0.909–1.061) | 0.067 |
| Tender joints at the start of treatment | 0.093 | 1.097 (0.831–1.449) | 0.513 | 0.157 | 1.170 (0.86–1.594) | 0.318 |
| Swollen joints at the start of treatment | 0.161 | 1.174 (0.898–1.536) | 0.241 | 0.171 | 1.186 (0.892–1.577) | 0.24 |
| VAS at the start of treatment | −0.72 | 0.487 (0.224–1.06) | 0.07 | −0.978 | 0.376 (0.147–0.96) | 0.041*** |
In the group of 23 patients starting using bDMARDs since the pandemic, higher the VAS score had an association with the longer retention time of the first bMARD. Age, number of swollen joints and number of tender joints at the beginning of treatment did not affect this retention time (Table 5).
Other factors related to the usage status of biologic drug therapies for rheumatoid arthritisWhen using the Cox regression model to determine the influence of factors on the overall and first-used bDMARD retention time, patients with moderate disease activity had a longer retention time than remission or low activity groups (HR=0.485 and 0.501, respectively, both p<0.05). Patients starting with TNF-α inhibitor had shorter overall and first-used bDMARD retention time than the group starting with IL-6 inhibitor (HR=1.545 and 2.419, respectively, both, p<0.05). No association was found with age, gender, place of residence, duration of disease, number of csDMARDs used, previous corticosteroid treatment, number of tender joints, number of swollen joints and VAS control at the start of treatment and the d retention time of overall and the first-used bDMARD (Table 6).
Factors related to the overall and first-used bDMARD retention time determined by Cox regression analysis.
| Related factors(p-value<0.05) | Overall retention time | First-used bDMARD retention time | ||||
|---|---|---|---|---|---|---|
| β | HR (95% CI) | p-Value | β | HR (95% CI) | p-Value | |
| Age at the start of treatment | ||||||
| <40 | 1 | 1 | ||||
| 40–59 | −0.431 | 0.65 (0.36–1.172) | 0.152 | −0.48 | 0.619 (0.354–1.082) | 0.092 |
| ≥60 | −0.279 | 0.756 (0.409–1.397) | 0.372 | −0.213 | 0.808 (0.45–1.45) | 0.475 |
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | −0.234 | 0.791 (0.497–1.26) | 0.324 | −0.145 | 0.865 (0.549–1.363) | 0.532 |
| Area | ||||||
| Rural | 1 | 1 | ||||
| Urban | 0.028 | 1.029 (0.742–1.426) | 0.865 | 0.084 | 1.088 (0.798–1.483) | 0.595 |
| Onset time | ||||||
| <6 months | 1 | 1 | ||||
| ≥6 months | 0.021 | 1.021 (0.668–1.562) | 0.922 | 0.052 | 1.053 (0.702–1.581) | 0.803 |
| Disease activity at the start of treatment | ||||||
| Remission or low | 1 | 1 | ||||
| Moderate | −0.724 | 0.485 (0.241–0.976) | 0.043*** | −0.691 | 0.501 (0.258–0.974) | 0.041*** |
| High | −0.55 | 0.577 (0.229–1.454) | 0.244 | −0.282 | 0.754 (0.309–1.843) | 0.536 |
| First-used bDMARD | ||||||
| IL-6 inhibitors | 1 | 1 | ||||
| TNF-α inhibitors | 0.435 | 1.545 (1.049–2.273) | 0.028*** | 0.883 | 2.419 (1.672–3.5) | <0.001*** |
| csDMARD at the start of treatment | ||||||
| 0 | 1 | 1 | ||||
| 1 | −0.3 | 0.741 (0.367–1.494) | 0.402 | −0.162 | 0.85 (0.438–1.65) | 0.631 |
| 2 | −0.147 | 0.863 (0.431–1.73) | 0.679 | −0.176 | 0.838 (0.434–1.62) | 0.6 |
| 3 | −0.629 | 0.5 (0.164–1.529) | 0.224 | −0.945 | 0.389 (0.13–1.166) | 0.092 |
| Corticoid at the start of treatment | ||||||
| No | 1 | 1 | ||||
| Yes | 0.252 | 1.286 (0.724–2.287) | 0.391 | 0.378 | 1.46 (0.855–2.494) | 0.166 |
| Tender joints at the start of treatment | 0.003 | 1.003 (0.958–1.05) | 0.896 | 0.896 | 0.896 | −0.019 |
| Swollen joints at the start of treatment | −0.012 | 0.989 (0.94–1.04) | 0.656 | 0.656 | 0.656 | −0.015 |
| VAS at the start of treatment | 0.131 | 1.141 (0.958–1.358) | 0.141 | 0.141 | 0.141 | 0.097 |
In this study cohort, the majority (85.4%) of patients were female and more than 90% of patients (91.8%) were over 40 years old. 1967 courses of treatment were examined, in which we counted 149 (7.6%) drug discontinuations, 760 (38.6%) dose tapering and 64 (3.3%) drug switches. Comparing drug usage status before and after COVID-19 pandemic appearance, retention time of biologic drugs, drug discontinuations and dose tapering increased after COVID-19 broke out, while the rate of drug switches was stable. Sex, kind of first bDMARD used, csDMARDs and corticoid usage status, disease activity levels were shown to be associated with retention time.
A total of 760 dose taperings were observed out of a total of 1967 bDMARD treatments. The main causes of the dose taperings include a good response (53.3%), economic impact (32.6%), impact of the COVID-19 pandemic (5.5%), and drug shortage (4.9%). In addition, 15 dose taperings were observed (2%) due to drug side effects, 11 dose taperings (1.4%) were due to no response to treatment (the patient adjusted the dose himself), 1 drug tapering was due to required surgery during the treatment and 1 was for unknown reasons. According to EULAR 2019 guidelines, if a patient is in sustained remission (reaching a mild disease activity level or less, usually in 6 months or more) after a glucocorticoid dose reduction, bDMARD dose reduction or tapering can be considered (however, bDMARD dose reduction or tapering should be applied before those of csDMARD).19 Regarding bDMARD dose reduction/tapering, according to ACR 2021 guideline, which is slightly different from EULAR 2019, when patients achieve sustained remission, csDMARD dose reduction/tapering should be made first rather than bDMARD dose reduction/tapering. Nevertheless, this recommendation is conditional and prioritizing bDMARD dose reduction or tapering can be applied based on patients’ and doctors’ choices considering factors such as cost of treatment, comorbidities as well as the risk of infection.20
Although there were a number of drug discontinuations in our study, which is actually dose tapering as prescribed by the doctor after reaching the treatment goal (dose tapering but the dosing interval is three times larger than the recommended interval), most of the patients stopped taking the drug on their own. The relationship between drug discontinuation and disease activity remains unclear.21,22 In a study by Sparks et al.26 on approximately 500 patients with various RA, the two main reasons for treatment discontinuation during the COVID-19 pandemic were fear of immunosuppression and the shortage of drugs, although the rate was not high. Among them, there were several patients had symptoms of COVID-19 infection. However, drug discontinuation was found not to be associated with RA exacerbation.23 In contrast, among 185 RA patients in a study of Tanaka et al. that reduced or discontinued RA drugs during the pandemic, 30.3% experienced a worse disease activity and most of them had to return to usual dosage.24 While another study in Japan has shown that arbitrary discontinuation of the drug can make the condition difficult to control even in cases where the patient has shown signs of clinical remission.25
There are several studies suggested that RA patients treated with bDMARDs, including rituximab, methotrexate, and other JAK inhibitors, had a higher risk of infection (from 1.5- to 2-fold) than those treated with csDMARDs.26,27 Cytokines storm with high levels of cytokines, including IL-6 and TNF in severe case of COVID-19 infection suggested the potential beneficial effect of IL-6 and TNF inhibitors therapy in preventing worsened cases. Thus, guidelines from the American College of Rheumatology suggested that in RA patients who experienced COVID-19, non-IL-6 and JAK inhibitors therapy should be temporarily stopped, while IL-6 inhibitor biologics may be continued.27 Since the lack of unified guidelines for RA treatment during COVID-19 pandemic, we have only described the frequency of extensions, but have not determined whether this dose tapering is correct according to current guidelines.28,29
In our study, the first-used bDMARD retention time was 34 weeks (95% CI: 23.51–44.49), shorter than some other studies in the world.30,31 This difference may be due to the relatively high number of subjects aged 60 and above in our study. They often have a weaker constitution and a weaker response to medication. Moreover, Vietnamese elderly often face inconvenience when moving to the hospital because most of them often move on their own without the help of their children. These reasons may cause them to make the decision to stop taking the drug earlier due to the inconvenience of traveling to hospital. The overall retention time of patients in our study was also quite short (49 weeks). This may be because of financial difficulties of patients in Vietnam, it is hard for them to afford the long-term treatment of bDMARDs. According to the World Bank report, Vietnam's GDP per capita in 2020 ranks 124th out of 196 countries and territories in the world.32 According to a 2011 systematic review, response to following bDMARD treatments is likely to decrease with a growing number of drug switches.33 In particular, the emergence of the COVID-19 pandemic has profoundly affected the employment and income status and even the lives of many patients, thereby leading to premature stopping or quitting. Thus, the short retention time of the first bDMARD in RA patients in Vietnam will increase the risk of having to change to another bDMARD and may lead to an increased rate of non-response in subsequent bDAMRDs treatments.
The results of the log-rank test showed that there was a statistically significant difference in the time to the probability of discontinuations before and during COVID-19 in terms of overall and first-used bDMARDs retention time (p=0.047, and 0.039, respectively). In addition, the rate of discontinuation and tapering since the outbreak of COVID-19 pandemic was significantly higher than before the pandemic with both p<0.001. This shows that COVID-19 affects the usage of bDAMRDs in RA patients. This result is similar to other studies around the world.34,35 It may be because during COVID-19 pandemic, the unemployment rate increased, people's incomes were reduced, so they could not maintain biologic drug treatment.36,37 However, more longitudinal studies are needed to clarify the long-term impact of COVID-19 pandemic.
Cox regression model was used to identify related factors. The group of 40–59 years old had a higher retention time of the first bDMARD than the under 40 years old group. A study by Brodszky et al. shown that age was increased with the prevalence of drug discontinuation, specifically, the HR increased by 0.7% with one additional year.38 However in this study, we found no difference in the retention time between 40–59 and ≥60 years of age groups, while the proportion of ≤40 was only accounted for 8.2% of studied subjects. This also can be explained by other factors related to patient's adherence, including local drug distribution, financial budget and degrees of disease activity. Regarding gender, our study was consistent with Brodszky et al.’s results that women had a higher retention time of the first bDMARD than men in the group taking the drug before the COVID-19. Patients with moderate disease activity had a higher retention time of the first bDMARDs than other groups. According to the results of several other studies, the influence of these factors on the retention time of biologic drugs is not uniform.5,17,31,39,40 The TNF inhibitors group had a shorter overall retention time and the first bDMARD retention time than the IL-6 inhibitors group in all patients and in patients treated before the pandemic and this result is similar to previous studies.17,41,42 The cost of treating RA with tocilizumab is significantly lower than with TNF inhibitors which may be a reason.43,44
Besides the obtained results, the study has some limitations. The group of patients before and during COVID-19 pandemic are two independent groups, so it is not possible to evaluate the change in the use of biologic drugs to treat RA by the pandemic in the same group of subjects. Besides, clinical characteristics of COVID-19 infection in patients were not involved in this study. Thus, it was hard to clarify the relationship between COVID-19 infection and the usage of biologic drugs in RA patients.
Currently, research on the effects and factors related to drug usage during COVID-19 pandemic is very limited. Therefore, we suggested that more longitudinal studies are needed to clarify the relationship between COVID-19 and drug usage as well as related factors.
ConclusionDuring COVID-19 pandemic, more than one third of courses of treatment were dose tapering (38%), while only 7.6% were drug discontinuation and 3.3% were drug switches. After COVID-19 pandemic broke out, drug discontinuations and dose taperings increased, but drug switches remained stable. There was a statistically significant difference in retention time before and during the pandemic in terms of overall retention time and retention time of the first bDMARDs. Four factors were proved to be associated with retention time including sex, kind of first bDMARD used, csDMARDs and corticoid usage status.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The research was approved by the Ethics Committee in Biomedical Research of Bach Mai Hospital according to the decision number: 2051/BVBM-HĐĐĐ.
Authors’ contributionsHai-Binh Bui, Hong-Thinh Lai: conceived and designed the experiments; performed the experiments; collected the data and informed consent; analyzed and interpreted the data; wrote the paper.
Thanh-Lam Nguyen, Thuy-Duong Vu, Nhat-Le Bui: analyzed and interpreted the data; wrote the paper; revised the paper.
Hai-Binh Bui, Hong-Thinh Lai, Van-Hung Nguyen, Thi-To-Chau Tran, Thi-Phuong-Thuy Nguyen, Thi-Ngoc-Lan Nguyen: collected the data and informed consent; materials, analysis tools; revised the paper.
Jaffar A. Al-Tawfiq: contributed analysis tools; analyzed and interpreted the data; revised the paper.
Dinh-Toi Chu: conceived and designed the experiments; analysis tools; analyzed and interpreted the data; wrote the paper; revised the paper.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors declare no conflict of interest.
We would like to thank for other members at Center for Biomedicine and Community Health, International School, Vietnam National University, Hanoi 100000, Vietnam for helping some parts in data analysis; and for critical reading and checking to improve the manuscript.
Lingvanex (free online version on lingvanex.com) was used for the translation of the manuscript from English into Spanish.