Autoinflammatory diseases are a group of disorders primarily characterized by dysregulation of innate immunity.1 Inflammasomes are complex protein structures that are formed either spontaneously or after a trigger and produce caspase 1. Caspase 1 cleaves pro-IL-1β and pro-IL-18 to IL-1β and IL-18.1,2 Cryopyrin-associated periodic fever syndromes (CAPS) are a group of autoinflammatory disorders caused by mutations in the NLRP3 gene.2 CAPS comprise three group of disorders: chronic infantile neurological cutaneous and articular syndrome (CINCA), also known as neonatal-onset multisystem inflammatory disease (NOMID), Muckle-Wells syndrome (MWS), and familial cold autoinflammatory syndrome (FCAS).1–4 CINCA is associated with the most severe phenotype in the spectrum of CAPS. The symptoms generally start in early infancy or even at birth. Fever, rash, arthropathy, central nervous system and ocular involvement are the main features of the disease. MWS is associated with moderate severity and characterized by attacks of fever, rash, arthralgia, arthritis and progressive sensorineural hearing loss. FCAS is considered as the least severe phenotype of CAPS and characterized by self-limited episodes of fever, rash, conjunctivitis, and arthralgia lasting less than 24h, precipitated by generalized exposure to cold. Some patients may develop daily rash suggesting chronic inflammation.2,4,5 The significance of V198M sequence variant in the NLRP3 gene is uncertain and considered as low penetrant mutation.6,7 Herein we present two cases at the extreme ends of the CAPS spectrum with V198M sequence variant in the NLRP3 gene to demonstrate that V198M sequence variant, even though regarded as low penetrant mutation, may cause to CINCA phenotype.
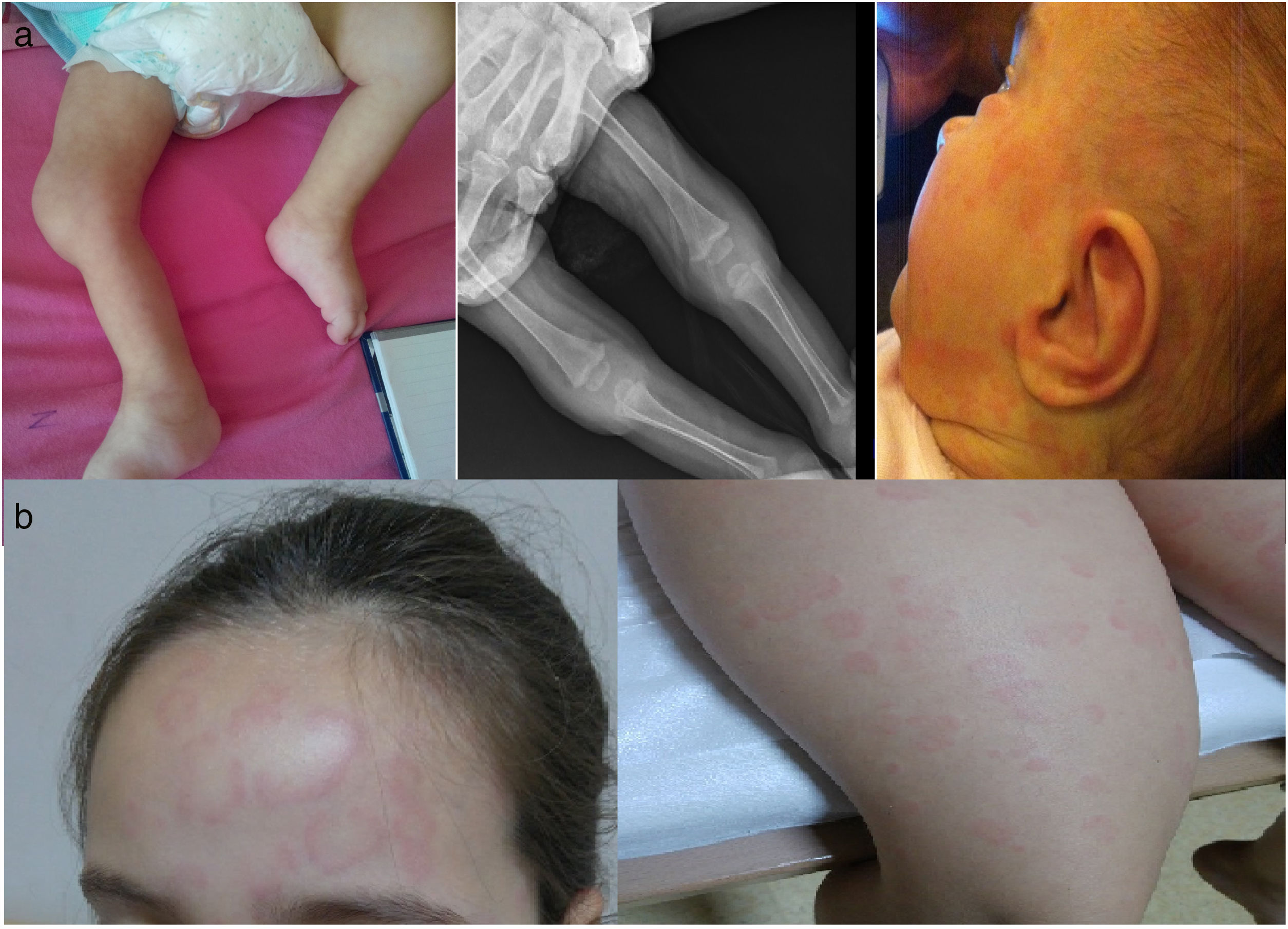
Case 1: A 16-month-old girl was consulted to pediatric rheumatology due to non-remitting fever for 3 weeks and persistence of high acute reactants (APR). She was born to non-consanguineous healthy parents at 29 weeks of gestation, 820g, and was hospitalized for 3 months postnatally. She had two episodes of persistent unexplained fever, high APR [C-reactive protein (CRP): 108mg/L, erythrocyte sedimentation rate (ESR): 88mm/h] with sterile cultures. She had never been hospitalized after discharge until this event but was followed closely because of prematurity and insufficient physical development. She had three episodes of fever and rash attacks and two episodes of only rash lasting 1–2 weeks in a year. On physical examination she had frontal bossing, saddle nose, nystagmus, swelling of bilateral knees and ankles (Fig. 1a) and high APR (CRP: 299mg/L, ESR: 140mm/h). Cranial magnetic resonance imaging, eye examinations, and auditory screening tests have been done at the follow-up visits for prematurity and all were normal. After combining the medical history and physical findings we have suspected CINCA and NLRP3 analysis showed heterozygous V198M sequence variant in exon 3. Anakinra 2mg/kg/day was started and APR dramatically improved with resolution of the fever. But after a few months, APR started to increase gradually again without fever and anakinra dose was increased up to 8mg/kg/day. Six months later anakinra was switched to canakinumab (2mg/kg/month) due to painful injections. Under canakinumab treatment, although at some intervals had APR near to normal levels, inflammation was not controlled totally (CRP: 51mg/L, ESR: 55mm/h) and dose was increased gradually up to 12mg/kg/month. She is being followed for 3.5 years and currently under monthly canakinumab (12mg/kg/month) injections with normal APR and without further attacks. Neurological development of the patient is compatible with her age.
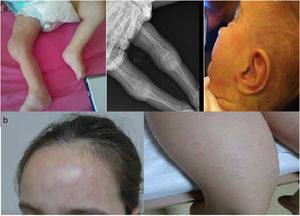
Case 2: An 8-year-old girl was admitted to clinic with the complaint of non-pruritic rash for the last 9 months. Parents were non-consanguineous and healthy. The rash was waxing and waning spontaneously but sometimes was getting worse with accompanying myalgia, red eyes and subtle fever for a few days. She had been followed and investigated by dermatology and pediatric allergy departments and many anti-histaminic medications have been tried with no effect on the rash. The family did not associate the start of the rash with cold exposure but recalled that she was feeling unwell with more rashes after exposure to cold. She had discrete annular urticaria-like rashes most notable on the forehead and lower extremities (Fig. 1b) with normal examination otherwise. APR was mildly elevated (CRP 18mg/L, ESR: 20mm/h). The clinical picture of the patient was compatible with FCAS and NLRP3 analysis showed heterozygous V198M sequence variant in exon 3. She is currently using canakinumab (2mg/kg) every two months for the last 3 years and did not have any further attack since the first dose of canakinumab.
In conclusion, although CAPS patients have the same genetic disturbance and pathophysiology, CINCA patients seem to have a continuous activity of inflammasome. Early initiation of treatment with an IL-1 blocker (anakinra, canakinumab or rilonacept) is essential to control ongoing inflammation and prevent secondary amyloidosis in CAPS patient and very high doses of IL-1 blockers may be needed in the very young to control the overwhelming inflammation.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThere is no conflict of interest associated with the manuscript.