Gaucher disease (GD), described in 1882 by Philippe Charles Ernest Gaucher, is a progressive, rare hereditary disease, with an autosomal recessive inheritance pattern.1 Included in the group of lysosomal storage diseases, it is characterized as being the most prevalent, with an estimated frequency of 1 per 50,000 to 1 per 100,000 population, with the exception of the Ashkenazi Jewish ethnicity in which the incidence is estimated to be 1 per 850 births.2,3 It produces a deficiency in the activity of the enzyme acid β-glucosidase (GBA), provoking an accumulation of glucocerebroside in the lysosomes of different cells,4 causing cytopenias, hepatosplenomegaly, changes in the central nervous system (CNS) and skeletal manifestations, the latter being one of the most disabling aspects. Depending on the clinical expression, different types can be distinguished: type 1 (adult non-neuronopathic), the most common form, occurring frequently in Ashkenazi Jews, with variable manifestations, and not involving the CNS; type 2 (acute neuronopathic), infrequent, with no ethnic-related dominance, fatal after birth and involvement of the CNS; and type 3 (subacute or chronic neuronopathic),4 beginning during childhood, adolescence or adulthood, with involvement of the CNS. Given the variety of conditions associated with bone pain, we consider it appropriate to report the case of our patient.
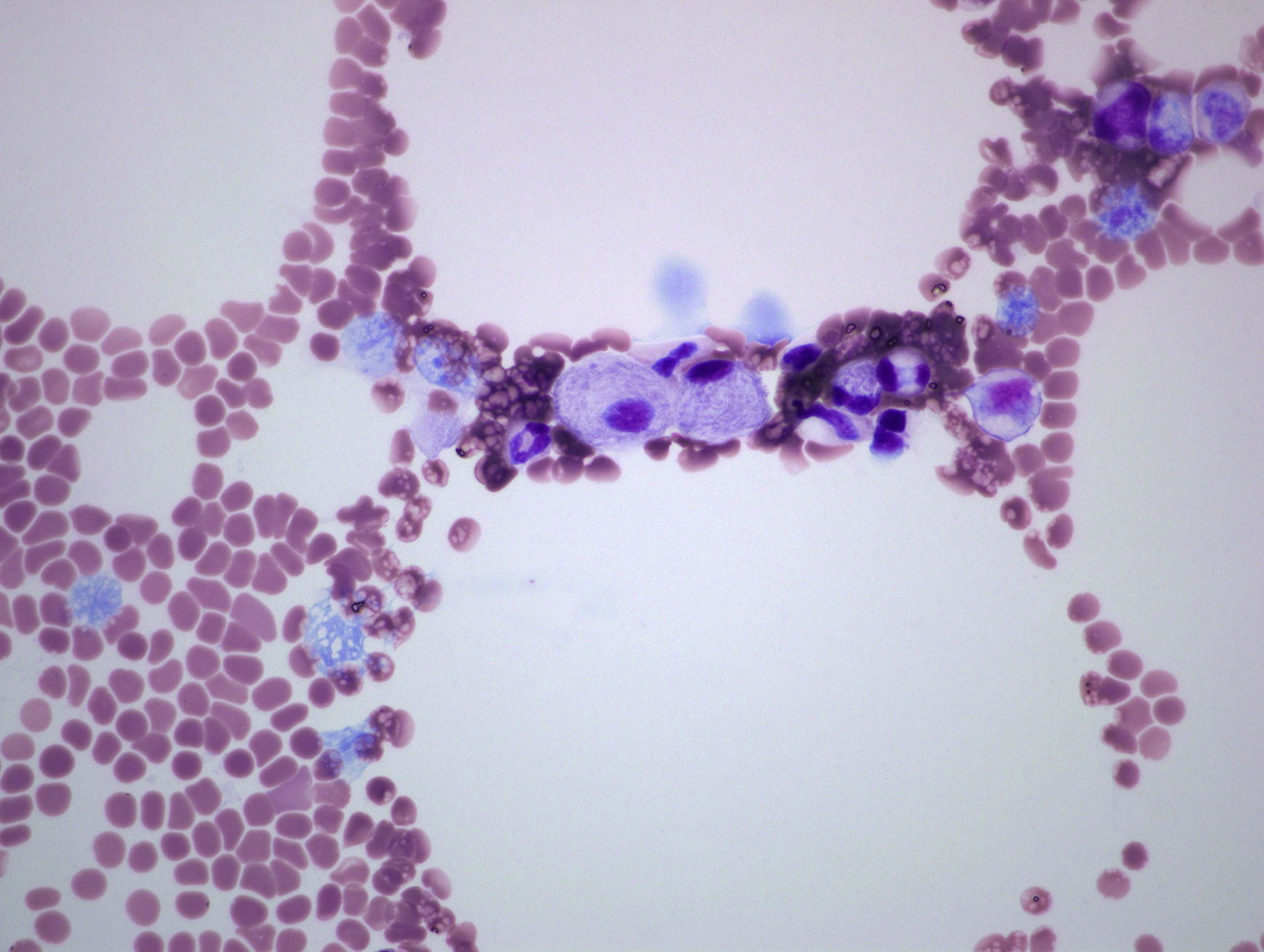
The patient was a 53-year-old man with no significant family or personal history. He was being studied because of neutropenia and thrombocytopenia with a duration of 10 years, as well as bone pain that had started 3 years earlier. He reported no hemorrhagic diathesis, infections or abdominal pain. Physical examination revealed splenomegaly, but the rest was normal. The results of laboratory tests included a leukocyte count of 3400mm3, neutrophils at 1200/mm3 and platelets at 93,000mm3; findings regarding hemoglobin, reticulocytes, coagulation, serum electrolytes, liver function, vitamin B12, folic acid, tumor markers (carcinoembryonic antigen, alpha-fetoprotein, CA 19-9, CA 15-3, prostate-specific antigen), β2-microglobulin, rheumatoid factor, erythrocyte sedimentation rate, antinuclear antibodies, immunoglobulins, protein profile and lymphocyte populations were normal. Serologic tests for hepatitis B and C viruses and human immunodeficiency virus were negative. Plain radiography of distal femur and thoracolumbar spine were normal. However, lumbar magnetic resonance showed a homogeneous hypointense signal in the vertebral bodies on T1 and T2-weighted sequences. Splenomegaly was confirmed by abdominal ultrasound and osteoporosis by bone densitometry (T-score femur: −2.9 standard deviations (SD); T-score spine: −2.8 SD). Bone marrow aspirate/biopsy (BMA/BMB) detected cells with eccentric nucleus, basophilic cytoplasm with the appearance of tissue paper, suggestive of Gaucher cells (GC) (Fig. 1). The GBA enzyme activity was determined in leukocytes by spectrofluorometry, which confirmed that it was lacking. The molecular genetic study showed double heterozygosity for the L444P and p.Tyr244Cys mutations.
This observation constitutes a representative example of the clinical, biochemical and genetic characteristics of type 1 GD.5 The fact that our patient had a history of years of bone pain can make diagnosis more difficult, when these manifestations are associated with other signs of the disease like cytopenias or organomegaly. Extra-articular manifestations are useful for reaching a correct diagnosis, avoiding incorrect diagnoses of inflammatory and/or autoimmune diseases. The BMA confirmed GD, which is accountable for an accumulation that generates substances responsible for bone resorption, producing pain, deformity and functional disability.6 Radiographic examination reveals manifestations such as abnormal bone remodeling (disclosing the Erlenmeyer flask deformity, which was not found in our patient), spontaneous fractures, osteopenia, osteonecrosis and osteolysis. However, the development of osteoporosis of unknown cause, whether or not it is associated with thrombocytopenia and splenomegaly, should lead us to suspect GD. These findings were significant and enabled the quantification of GBA activity, and led us to request a genetic study to confirm the disease.6 A high index of suspicion and initial biochemical studies are needed to verify the diagnosis and begin enzyme replacement therapy to revert, establish and improve the clinical prospects of the patient.
Please cite this article as: Herráez-Albendea MM, Fernández-Cofrades EG, Jarilla-Fernández MC, Jiménez-Burgos F. Enfermedad de Gaucher: a propósito de un caso. Reumatol Clin. 2017;13:242–243.