It has been shown that no anti-TNF biologic drug inhibits or retards radiographic progression at 2 years in patients with ankylosing spondylitis (who met the New York criteria) when compared with historical cohorts such as OASIS.1–3 There are no published studies evaluating the effect of biologic therapy on structural progression in patients with non-radiographic axial spondyloarthritis. In studies evaluating radiological progression, patients do not receive anti-TNF-α or do it in a very small percentage. In 2 recent GESPIC cohort studies that assess the progression in the spine and sacroiliac joints of 95 patients with non-radiographic axial spondyloarthritis, only one patient received biological therapy. In these studies, 10.5% of patients showed progression of sacroiliitis and 7.4%, had spinal progression at 2 years follow-up.4,5
The aim of this study was to evaluate the radiological progression in spine and sacroiliac joints at 2 years in a cohort of patients with non radiographic axial spondyloarthritis treated with anti-TNF-α.
After a systematic review of medical records, we selected all patients with non radiological axial spondyloarthritis in our service and biological treatment with anti-TNF drugs at a standard dose for a minimum of two years and who had a baseline simple X-ray of the spine (cervical and lumbar lateral projection) and pelvis (anteroposterior view), and a follow-up X-ray 2 years later under the same treatment.
All selected patients, 19, fulfilled the ASAS classification criteria for axial spondyloarthritis,6 but did not meet the New York radiological criteria for ankylosing spondylitis7 (bilateral sacroiliitis at least grade 2 or unilateral grade 3–4).The 19 patients met the 2 entrnace criteria (back pain ≥three months and age of onset <45 years), 13 (65%) met the HLAB27 criteria and 6 (35%) the imaging, and thus, had acute inflammation on MRI, indicating sacroiliitis.
We excluded patients with cutaneous psoriasis and inflammatory bowel disease.
Twelve patients received adalimumab, 2 infliximab, and 5 etanercept. Only 2 patients (10.5%) received concomitant therapy with NSAIDs for 3 or more months.
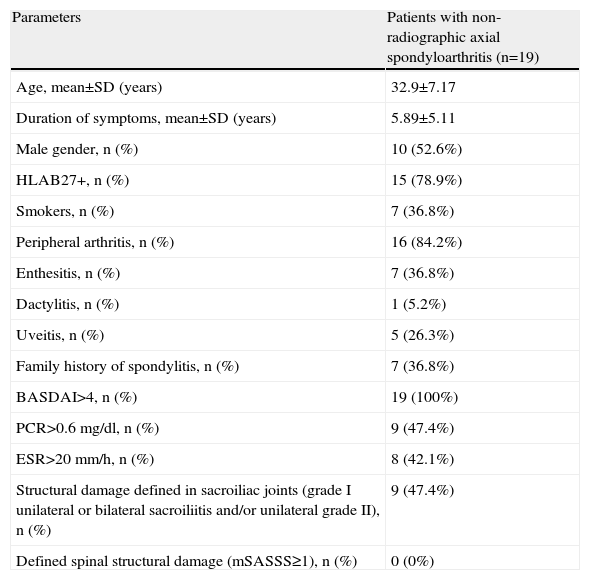
The baseline characteristics of the patients are shown in Table 1.
Baseline Characteristics of Patients With Non-Radiographic Axial Spondyloarthritis.
| Parameters | Patients with non-radiographic axial spondyloarthritis (n=19) |
| Age, mean±SD (years) | 32.9±7.17 |
| Duration of symptoms, mean±SD (years) | 5.89±5.11 |
| Male gender, n (%) | 10 (52.6%) |
| HLAB27+, n (%) | 15 (78.9%) |
| Smokers, n (%) | 7 (36.8%) |
| Peripheral arthritis, n (%) | 16 (84.2%) |
| Enthesitis, n (%) | 7 (36.8%) |
| Dactylitis, n (%) | 1 (5.2%) |
| Uveitis, n (%) | 5 (26.3%) |
| Family history of spondylitis, n (%) | 7 (36.8%) |
| BASDAI>4, n (%) | 19 (100%) |
| PCR>0.6mg/dl, n (%) | 9 (47.4%) |
| ESR>20mm/h, n (%) | 8 (42.1%) |
| Structural damage defined in sacroiliac joints (grade I unilateral or bilateral sacroiliitis and/or unilateral grade II), n (%) | 9 (47.4%) |
| Defined spinal structural damage (mSASSS≥1), n (%) | 0 (0%) |
Two trained readers, JL and MA, examined the spinal radiographs according to the Stoke Ankylosing Spondylitis Spine Score (mSASSS)8 and the sacroiliac joint x rays according to the grading system of the modified New York criteria for ankylosing spondylitis7 in chronological order (baseline and follow-up at 2 years with the same biological treatment).
The intraclass correlation coefficients (ICC) for baseline rates were 1 in mSASSS and 0.4 index in grading the sacroiliac index and the ICC for the change rates were 1 in both reading systems.
The mSASSS index was zero units at baseline and at 2 years of treatment in all patients (none developed structural lesions in the spine).
There was no sacroiliac radiological progression in any patient. None of the 19 patients in the cohort met the New York criteria for ankylosing spondylitis after 2 years of biological therapy with anti-TNF-α⋅
In conclusion, none of the patients in our cohort with non radiographic axial spondyloarthritis showed radiographic progression in the spine or sacroiliac joints after 2 years of treatment with anti-TNF, unlike other non-radiological spondylitis cohorts without biological therapy.
In the first GESPIC cohort study, assessing sacroiliac radiographic progression at 2 years, the only predictor of radiographic progression were baseline CRP levels.4 In our cohort, 47.4% of patients had CRP levels greater than 0.6mg/dl. In this study by Poddubnyy et al.,4 the high level of CRP was also a significant predictor of progression of non-radiological spondylitis to ankylosing spondylitis and the presence of structural damage defined at baseline was also associated with greater progression to ankylosing spondylitis, although not statistically significant. In another study, Huerta-Sil et al.,9 also found that low-grade sacroiliitis was a prognostic factor for the development of spondylitis. In our cohort, 47.4% of patients had low-grade structural damage (grade I unilateral or bilateral sacroiliitis and/or unilateral grade II).
In the second GESPIC5 cohort study evaluating spinal radiographic progression at 2 years, only the presence of basal syndesmophytes was a statistically significant predictor of progression in the non radiological spondylitis group, although there were was radiological progression in patients with no baseline syndesmophytes (the vast majority).
In our cohort of patients, although they presented no baseline syndesmophytes, the fact that they showed no radiographic spinal or sacroilliac progression indicates that anti-TNF therapy may inhibit or delay progression in patients with axial non-radiological ankylosing spondylitis, although further studies are necessary with control groups and more patients. This data indicates the existence of a window of opportunity in which an effective treatment may alter the course of disease.
Please cite this article as: Almirall M, López-Velandia JG, Maymó J. Ausencia de progresión radiológica a los 2 años en una cohorte de pacientes con espondiloartritis axial no radiológica tratados con terapia anti-TNF-α. Reumatol Clin. 2014;10:134–135.